If you enjoy this content, please share it with a colleague
Philips
RELATED CONTENT
Royal Philips Electronics is introducing three new imaging systems to help radiology departments increase both the number of patients and range of clinical exams they can handle. Showcased at the 2013 European Congress of Radiology (ECR 2013), all three systems combine the capabilities for high patient throughput with the functionality and advanced clinical imaging applications.
Advances in digital mammography are evolving as clinicians look to improve breast cancer screening in women. In recent years, new technological trends have emerged; notably, the addition of 3-D breast imaging, or tomosynthesis, which was approved by the U.S. Food and Drug Administration (FDA) in 2011. But as technology improves, questions remain over the recommended frequency of screening for breast cancer.
Increasing research on women with dense breasts is having a positive effect on imaging modalities. It is estimated that between 30–40 percent of women in the United States have dense breasts. Because fatty breast tissue makes it difficult to identify breast tumors on standard mammography exams until very advanced stages, dense breasts are a strong independent factor for breast cancer. Research has shown that it is beneficial for this population of women to invest in additional imaging.
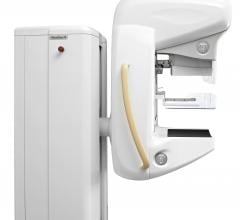
Royal Philips Electronics announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its MicroDose SI system, the first full-field digital mammography (FFDM) system on the market with the capability to enable future Single-Shot Spectral Imaging applications*. Philips is working on future software applications like Spectral Breast Density Measurement*, which will build upon the MicroDose SI technology.
Royal Philips Electronics announced it received 510(k) clearance from the Food and Drug Administration (FDA) for its MicroDose SI system, the first full-field digital mammography (FFDM) system on the market with the capability to enable future Single-Shot Spectral Imaging applications*. Philips is working on future software applications like Spectral Breast Density Measurement*, which will build upon the MicroDose SI technology.
Neusoft Corporation announced that it has signed a Term Sheet with Philips on the proposed transaction of shares and assets in Philips and Neusoft Medical Systems Co. Ltd. ("PNMS"). According to the Term Sheet, Neusoft's wholly owned subsidiary Neusoft Medical System Co. Ltd. and its overseas associates intend to acquire 51 percent equities in PNMS held by Philips. Upon the completion of the proposed transaction, all intellectual property rights of PNMS will be shared by both Neusoft and Philips. In addition, a team of approximately 100 to 150 computed tomography (CT) system and component engineers and supporting staff will transfer from the joint venture to Philips.
Since my first RSNA meeting in 1984, I had been told the only radiologists who had patients were those who did interventions. The mainstream radiologist was a physicians’ physician, providing expert interpretation of medical images, identifying the subtle visual indicators of disease and ruling out diagnoses when signs of pathology were absent. This year was different.
The Radiological Society of North America (RSNA) annual meeting has transitioned in recent years from an imaging device focus to an imaging information technology focus. The interest in software continued at this year’s meeting, partly fueled by the need for healthcare organizations to meet Stage 1 and 2 meaningful use requirements. Two key trends seen throughout the show floor included remote viewing systems for radiology images and technology streamlined to aid workflow efficiency.
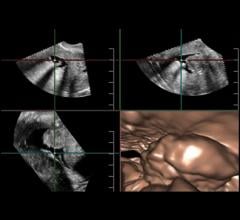
With ever-increasing concerns about radiation dose and the appropriate use of diagnostic imaging tests, doctors are continuously looking for ways to better image their patients. Advances in ultrasound are making the technology appear more attractive for certain clinical applications, from breast health to cardiology, thanks to their noninvasiveness, cost-effectiveness and lack of radiation. Some of the emerging innovations in ultrasound, such as real-time 3-D imaging and the development of wireless transducers, are set to keep the market going throughout 2013 and beyond.
Just a few years ago, the debate in radiology departments about workhorse X-ray systems was whether to convert from analog film to computed radiography (CR) cassettes and digital readers, or to direct imaging digital radiography (DR) systems. Today, there is no doubt DR has won that debate and is being widely adopted, and CR is falling out of favor.

 April 01, 2013
April 01, 2013