April 24, 2012 —ActiViews Inc. announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its flagship product, CT-Guide Needle Guidance System, for liver interventions.
April 24, 2012 — Novarad has introduced a cloud-based version of its picture archiving and communication system (PACS), NovaPACS Express, specifically for small medical offices with imaging capability.
April 23, 2012 — Using a special piece of MRI (molecular resonance imaging) equipment, doctors from the University of Missouri College of Veterinary Medicine (MU) were able to remove a dangerous tumor from a beloved pet and therapy dog.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
April 23, 2012 — Patients at St. James’s University Hospital in Leeds, United Kingdom, were the first to benefit from the use of Agility, the latest multileaf collimator (MLC) from Elekta.
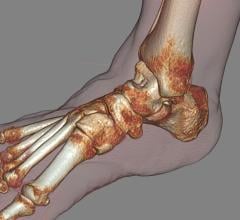
April 23, 2012 — CurveBeam announced it has received 510(k) approval from the U.S. Food and Drug Administration (FDA) for pedCAT, its weight-bearing, in-office 3-D foot and ankle scanner.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
April 23, 2012 — A conclusion reached in a University of North Carolina study was contradicted by a number of peer-reviewed studies that found protons reduce – not increase – gastrointestinal (GI) side effects.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
April 23, 2012 - Records exposing the level of medical device recalls made in the United States, and therefore the multitude of serious health risks patients have been subjected to over the past few years, have sparked calls for stricter regulations, according to a new report by healthcare intelligence company GBI Research.
April 20, 2012 — The National Institutes of Health (NIH) awarded a sole source contract for a radiology information system (RIS) to Carestream Health.
April 20, 2012 — GE Healthcare and NXT2B, a privately owned venture capital company, announced they have entered into a joint financing agreement with the goal of developing a micro-scale radiotracer infrastructure including cyclotron and positron emission tomography (PET) tracer production. The three-year development project will be led by GE Healthcare and will be headquartered in Uppsala, Sweden. The terms of the agreement were not disclosed.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
April 20, 2012 — Guided Therapeutics Inc. announced this week that the LuViva Advanced Cervical Scan is being introduced to medical leaders at the 40th Anniversary Meeting of the British Society for Colposcopy and Cervical Pathology (BSCCP) in Gateshead, United Kingdom, by Eurosurgical Ltd., the company’s U.K. distributor.
Over the past several years, the radiology community has taken it on the chin with reimbursement cuts to reports of radiation overexposure. Earlier this month, it looked like radiology would be hit again, this time by nine physician societies whose leaders compiled a list of “five things physicians and patients should question” – part of a concerted “Choosing Wisely” campaign launched by organized medicine.
April 19, 2012 — GE Healthcare this week announced it has received U.S. Food and Drug Administration (FDA) clearance of Q.Freeze, one of the positron emission tomography/computed tomography (PET/CT) quantitative imaging technologies designed to enable treatment evaluation earlier in a patient’s cancer treatment.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
April 19, 2012 — Carestream Healthcare announced it received U.S. Food and Drug Administration (FDA) clearance for its Carestream DRX-Revolution Mobile X-Ray System, and is now accepting orders from U.S. healthcare facilities.
April 19, 2012 - The FDA received a report from a hospital that 16 patients had developed hospital-acquired infections with the bacteria Pseudomonas aeruginosa following an examination with transesophageal echo (TEE) ultrasound probes using Other-Sonic Generic Ultrasound Transmission Gel. Upon investigation, theultrasound gel was found to be contaminated with the bacteria Pseudomonas aeruginosa and Klebsiella oxytoca. Manufactured by Pharmaceutical Innovations Inc., the non-sterile gel is used in ultrasound procedures to improve the transmission of the ultrasound signal from the transducer to the body.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
April 17, 2012 -- Two hospitals announced treating their first patients with the Curve Image Guided Surgery system by Brainlab, which offers a command and control center for information-guided surgery.
April 17, 2012 - NoMoCo Inc.'s 12-piece pillow support system products optimize brain images for accurate, detailed diagnostic reports by the neuroradiologist in research centers, hospitals or clinical settings. The pillows are precision cut, designed from high-grade memory foam, easily cleanable and artifact free. NoMoCo helps patients complete radiologic exams with maximum comfort and with minimal movement, eliminating the need for repeat tests.
April 17, 2012 — Radius LLC is expanding its unique pay-per-study RIS/PACS model to include the option of on-demand 3-D imaging reconstruction.
April 17, 2012 - On April 9, 2012, the FDA's Center for Devices and Radiological Health (CDRH) launched its second version of the Innovation Pathway, called "Innovation Pathway 2.0." This program offers new and modified tools and methods to deepen collaboration between the FDA and innovators early in the process, prior to pre-market submission, with the goal of making the regulatory process more efficient and timely.
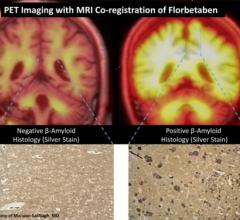
April 16, 2012 — Piramal Healthcare Ltd. has signed an agreement to acquire worldwide rights to the molecular imaging research and development portfolio of Bayer Pharma AG through its newly created subsidiary, Piramal Imaging SA. The portfolio includes rights to florbetaben, which is in the final stages of its Phase III clinical trials.
April 16, 2012 — Medic Vision Imaging Solutions Ltd. announced that within six months of clinical use in the United States, its SafeCT image enhancement system has delivered diagnostic image quality to more than 20,000 CT (computed tomography) studies acquired with low-dose protocols.

 April 24, 2012
April 24, 2012