May 18, 2012 — The U.S. Food and Drug Administration (FDA) has published a draft guidance document entitled "Pediatric Information for X-ray Imaging Device Premarket Notifications." The document encourages manufacturers to consider the safety of children in the design of new X-ray imaging devices.
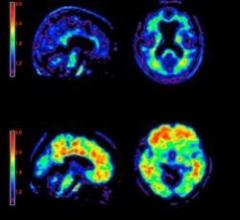
May 18, 2012 — In April the U.S. Food and Drug Administration (FDA) approved Avid Radiopharmaceuticals’ (now part of Eli Lilly) Amyvid, a brain plaque diagnostic tool using florbetapir (F-AV-45). This radiotracer agent is delivered intravenously and is drawn to beta-amyloid plaque, a characteristic of AD.
May 18, 2012 - New research from the University at Buffalo suggests that cardiologists may have a new way to identify patients who are at the highest risk of sudden cardiac arrest, and the most likely to benefit from receiving an implantable cardiac defibrillator (ICD).
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
May 18, 2012 — DICOM Grid, provider of a cloud software-as-a-service (SaaS) platform for medical imaging applications, this week announced the close of a $5 million financing expansion round led by Canaan Partners, CHL Medical Partners and existing individual investors.
May 17, 2012 — Viztek announced an upgrade to its Opal-Ortho PACS (picture archiving and communications system) solution for orthopedic practices. The solution offers one single appliance that controls every aspect of orthopedics imaging, integrating the digital radiography (DR) or computed radiography (CR) solution and the PACS for optimal efficiency.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
It was set a decade ago, a battle between the upstart and the champ. It wasn’t the first time. The standard bearer for magnetic resonance imaging (MRI) had stood its ground many times before, facing down ultra-low, low and mid-field cylindricals. Even a 2T took a shot. Low- and mid-field opens and ones with “high-field” tendencies had theirs, as well. But tried and proven 1.5Ts always came through.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
May 16, 2012 — Steinberg Diagnostic Medical Imaging Center (SDMI) in Las Vegas has installed the first Aquilion Prime 160 series from Toshiba America Medical Systems Inc. in the United States.
May 16, 2012 — Philips Healthcare has established a five-year agreement with St. Joseph’s Hospital and Medical Center in Phoenix to pursue research to help accelerate the advancement of magnetic resonance imaging (MRI) technology.
May 16, 2012 — A large multicenter study found that the Breast Imaging and Reporting Data Systems (BI-RADS) terminology used by radiologists to classify breast imaging results is useful in predicting malignancy in breast lesions detected with magnetic resonance imaging (MRI).
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
May 16, 2012 — David Fisher, vice president for healthcare policy and strategy at Siemens Healthcare, testified on behalf of the Medical Imaging & Technology Alliance (MITA) today at the Internal Revenue Service (IRS) Hearing on Proposed Medical Device Excise Tax Regulations [REG-113770-10] to voice the industry’s concerns about implementation of the tax imposed by the Affordable Care Act. MITA has submitted comments to the IRS on the proposed regulations, slated to go into effect in 2013, requesting clarification surrounding how and when the new tax will be put into practice.
Single photon emission computed tomography (SPECT) remains a well-entrenched imaging modality for nuclear myocardial perfusion imaging (MPI) more than 30 years after its introduction. Due to SPECT’s reliability, cost-effectiveness and the wealth of data showing its clinical validation, it remains more common in MPI than its competition, positron emission tomography (PET).
Hardware and software advances are enabling echocardiography to greatly expand its capability with increased quantification accuracy, ease-of-use, increased workflow efficiencies and wider use outside of echo labs. Today, cardiovascular ultrasound systems are being integrated into point-of-care for triage, and in operating rooms and cath labs for procedural guidance to cut the use of contrast and ionizing radiation.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
May 15, 2012 -- Viztek, a digital imaging solution provider, announced the availability of Opal-Mammo, a complete women's health solution with the industry's leading combination of a Web-based mammography PACS with integrated tracking software.
May 15, 2012 -- European cancer experts have reported their progress with promising new radiosurgical techniques for treating cancer and other conditions using new linear accelerator technology from Varian Medical Systems. Speakers at Varian's Emerging Technologies Symposium at the annual ESTRO conference detailed their experiences with fast hypo-fractionated treatments for prostate patients and RapidArc Radiosurgery for intracranial and central nervous system indications.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
May 15, 2012 - Breast brachytherapy with a strut-based applicator is a well-tolerated and effective treatment for early-stage breast cancer, according to a multi-site study with a median follow-up of four years. The research was presented as a scientific poster at the European Society for Radiotherapy & Oncology (ESTRO) World Congress of Brachytherapy in Barcelona, May 10-12.
May 15, 2012 – International medical technology company, Sectra, announced an agreement to distribute MagView mammography reporting, mammography tracking and compliance software fully integrated with Sectra Breast Imaging PACS.
May 14, 2012 — On May 9, the U.S. Food and Drug Administration announced that it is seeking public comment on a proposal encouraging manufacturers to consider the safety of children in the design of new X-ray imaging devices. In the draft guidance, FDA is recommending that manufacturers design new X-ray imaging devices with protocols and instructions that address use on pediatric patients.
Two next-generation automated contrast injector systems were recently introduced in the United States.
Medical devices employed for injecting radio-opaque contrast media into the body to enhance the visibility of tissues for a medical imaging procedure are known as contrast injectors. Over the years, with ever-increasing technological sophistication, these contrast injectors have evolved from manual injectors having manifolds with stop-cocks to automated versions, providing added accuracy and advantages. These automated versions are known as autoinjectors. Autoinjectors are capable of controlling the amount of contrast media injected, utilization rate, and are also able to increase dosage to keep pace with fast medical imaging scanners as well as alert the physician of potential hazards, such as air embolisms or extravasations.
May 15, 2012 — Varian Medical Systems is showcasing a new user interface for its ProBeam proton therapy system at the annual meeting of the Particle Therapy Co-Operative Group (PTCOG 51) taking place May 14-19 in Seoul, Korea.

 May 18, 2012
May 18, 2012