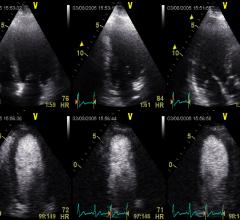
August 28, 2012 — The International Contrast Ultrasound Society (ICUS) applauded the U.S. Food and Drug Administration’s (FDA) decision to modify the U.S. product label for several ultrasound contrast agents used to improve the accuracy of radiation-free ultrasound scans.
August 28, 2012 — GE Healthcare announced important changes to the U.S. product label for Optison (perflutren protein-type A microspheres injectable suspension, USP), a contrast agent that may improve visualization of the left ventricular border, an area of the heart that is critical to see in order to diagnose certain heart diseases such as hypertrophic cardiomyopathy.
Dr. Gary Ezzell, president of the American Association of Physicists in Medicine (AAPM), talks about current trends in ...
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
August 28, 2012 — NDS Surgical Imaging (NDSsi) is now shipping its new Dome GX4MP radiology display, a 30-inch widescreen model offering multimodality viewing in both color and grayscale.
Web Stayman, Ph.D., Johns Hopkins University, presents an overview of research he presented at the 2012 American ...

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Dr. Sabee Molloi from the School of Medicine at the University of California, Irvine, worked with a team on a study ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
August 27, 2012 — Varian Medical Systems, a manufacturer of technology for the treatment of cancer with radiotherapy and radiosurgery, has launched Portuguese versions of its software systems for planning and managing radiotherapy treatments. Varian personnel worked with Brazilian cancer treatment specialists to translate its software products into Portuguese and also to adapt them so that they facilitate Brazilian treatment practices, in order to support the safe and effective use of radiotherapy in the country.
August 27, 2012 — Siemens Healthcare announced that the company has entered into a definitive agreement to acquire substantially all of the assets of Penrith Corp. of Plymouth Meeting, Pa., a manufacturer of integrated ultrasound imaging systems. Through this acquisition, Siemens will offer new and improved diagnostic capabilities. The deal is expected to close in September 2012.
August 27, 2012 — A recent multi-center study, led by researchers from Wake Forest School of Medicine and published in the Journal of the American Medical Association (JAMA) has indicated that computed tomography (CT) scanning of the heart and measuring the coronary artery calcium (CAC) score is the most accurate predictor of cardiovascular disease for individuals at intermediate risk for heart disease.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
August 27, 2012 — The growth rate of positron emission tomography (PET) and PET/computed tomography (CT) medical imaging scans in the United States began to slow in 2011, with an estimated 1.85 million clinical PET patient exams performed in 2011, down from a double-digit growth rate reported in previous years, according to a new market research report by IMV Medical Information Division.
August 27, 2012 — Positron Corp., a molecular imaging healthcare company, announced the submission of a drug master file (DMF) with the U.S. Food and Drug Administration (FDA) for the production of active pharmaceutical ingredient- (API) grade strontium-82 through its wholly owned subsidiary, Manhattan Isotope Technology LLC (MIT).
There are some key requirements specifically aimed at imaging and radiology included in the Stage 2 meaningful use requirements released Aug. 23 by the Department of Health and Human Services (HHS). HHS fielded many concerns and questions about these requirements and offered answers in the 642-page document.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
August 22, 2012 — In a head-to-head contest between the New Orleans Criteria (NOC) and the Canadian Computed Tomography (CT) Head Rules (CCHR), the Canadian rules showed higher sensitivity and specificity in predicting neurosurgical intervention for mild head injury. The results of the study, conducted in Tunisia, were published online August 21, in Annals of Emergency Medicine ("Prediction Value of the Canadian CT Head Rule and the New Orleans Criteria for Positive Head CT Scan and Acute Neurosurgical Procedures in Minor Head Trauma: A Multicenter External Validation Study").
August 24, 2012 — On August 20, 2012, InSightec announced it received approval from the U.S. Food and Drug Administration (FDA) to begin Phase I clinical trials evaluating the use of its ExAblate Neuro system for the treatment of patients with tremor-dominant Parkinson’s Disease (PD). This device is the first clinical system to use magnetic resonance- (MR) guided focused ultrasound (MRgFUS) through an intact skull, offering noninvasive transcranial treatment without any incisions or ionizing radiation.
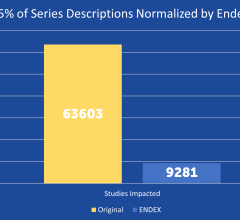
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
August 24, 2012 — The Stage 2 meaningful use requirements for electronic health records (EHRs) were released Aug. 23 by the Department of Health and Human Services (HHS) and the Centers for Medicare and Medicaid (CMS). The rule specifies criteria that eligible professionals (EPs), eligible hospitals and critical access hospitals (CAHs) must meet in order to qualify for CMS EHR incentive payments. In addition, it specifies payment reductions for EPs and facilities failing to demonstrate meaningful use of certified EHR technology.
August 24, 2012 — West Physics Consulting, a national provider of medical and health physics consulting and testing services, has begun offering computed tomography (CT) dose reduction, fluoroscopy dose reduction and related services to medical imaging facilities throughout the United States.
August 23, 2012 — Varian Medical Systems is establishing an in-country subsidiary in South Korea. Varian Medical Systems Korea, based in Seoul, will support the launch and rollout of the company's TrueBeam treatment system while, over time, providing a local base for all the company's business units.
August 24, 2012 — On October 24, 2012, the U.S. Food and Drug Administration's Radiological Devices Panel of the Medical Devices Advisory Committee will discuss, make recommendations and vote on a premarket approval (PMA) application supplement to expand the indications for use of the Selenia Dimensions 3-D system with C-View software module, sponsored by Hologic Inc.
August 23, 2012 — The U.S. Food and Drug Administration (FDA) has cleared Toshiba’s Aquilion RXL Edition CT system. The system reconstructs images faster and includes the latest dose reduction technologies, providing faster, safer information to physicians and patients.
August 21, 2012 — IBA (Ion Beam Applications S.A.) officially opened its International Competence Center (ICC) in July in Nuremberg, Germany. The ICC provides high-level practical training to promote safer radiation therapy to healthcare professionals, using the latest software and hardware radiation treatment and specialist measurement tools.

 August 28, 2012
August 28, 2012