If you enjoy this content, please share it with a colleague
Philips
RELATED CONTENT
When 64-slice computed tomography (CT) systems were introduced nearly a decade ago, they were purchased in large numbers ...
Computed tomography (CT) has enjoyed a place of prominence in medical imaging since its creation in the 1970s. The ...
Significant national and international momentum is building to include a proton therapy unit as part of the planned expansion of SAHMRI, South Australia’s Health and Medical Research Institute. It would be the first facility in the Southern Hemisphere to deliver proton therapy.
In an effort to demonstrate how 3-D printing can help better interpret medical imaging, Philips teamed up with 3-D printer manufacturer Ultimaker to create detailed replicas of Philips magnetic resonance imaging (MRI) scans. The replicas were displayed at the 2015 International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting in Toronto, May 30-June 5.
Philips and Profound Medical Corp., a Toronto-based medical device company, announced a joint development agreement to support Profound Medical's proprietary TULSA (Transurethral Ultrasound Ablation) technology on Philips' Ingenia and Achieva 3T magnetic resonance imaging (MRI) systems. The technology is designed to treat patients with prostate cancer
DAIC Editor Dave Fornell shares some of the most innovative new technologies shown on the expo floor and discusses in ...
Image-guided radiation therapy (IGRT) affords greater accuracy of dose distribution during cancer treatment, allowing the radiation oncologist to see how a tumor is responding over the course of treatment. This has traditionally been accomplished with computed tomography (CT) or X-ray scans, which requires extra radiation exposure for the patient, with relatively poor contrast in soft tissue due to uniform electron density. Since treatment is only as good as the images provided, efforts are under way to find a better modality — and magnetic resonance imaging (MRI) may hold the answer.
The U.S. Food and Drug Administration (FDA) announced its latest efforts in supporting the Bonn Call for Action on radiation protection.
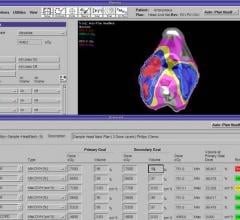
Pinnacle³ Auto-Planning from Philips is designed to simplify and accelerate intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) planning.
Philips announced the introduction of Lumify, its first app-based ultrasound solution that will extend the reach of ultrasound applications across the health continuum using mobile technology. The technology was unveiled at the Social Media and Critical Care (SMACC) conference, June 23-26, in Chicago.


 September 09, 2015
September 09, 2015