If you enjoy this content, please share it with a colleague
Philips
RELATED CONTENT
As one of the largest Catholic, not-for-profit healthcare systems in the United States, St. Louis-based SSM Health has expanded immensely over the years. So, too, has its ability to apply innovative imaging solutions that meet the needs of it growing patient base. One of the largest integrated delivery systems in the nation, SSM Health serves the comprehensive health needs of communities across the Midwest, with facilities in Illinois, Missouri, Oklahoma and Wisconsin.
Philips and Hologic, Inc. announced a global partnership agreement to offer care professionals integrated solutions comprising diagnostic imaging modalities, advanced informatics and services for screening, diagnosis and treatment of women across the world. The collaboration combines Hologic's innovative mammography technologies and Philips' leading portfolio of ultrasound, MRI, CT and X-ray systems, advanced informatics and broad range of services, including maintenance, upgrade, training and operational performance management services.
ITN Contributing Editor Greg Freiherr offers an overview of artificial intelligence advances at the Radiological Society of North America (RSNA) 2017 annual meeting.
January 17, 2018 — Philips recently announced the launch of Technology Maximizer, a cross-modality program designed to ...
Exhibitors at the 2017 Radiological Society of North America (RSNA) meeting rode the artificial intelligence (AI) bandwa ...
Philips and Arizona-based Banner Health recently announced they have extended their multi-year partnership to include adoption of Philips' PerformanceBridge Practice, helping Banner to accelerate its clinical transformation in radiology and further deliver on its goal of improving care for patients. PerformanceBridge Practice is a part of the Philips PerformanceBridge portfolio, a suite of operational performance improvement software and services that assist radiology departments in enhancing productivity, improving the patient and staff experience, while delivering better value-based care. Philips and Banner will work collaboratively to pinpoint improvement opportunities, and a dedicated Philips Solution Advisor will help Banner to develop solutions that address areas for improvement. Banner will roll out PerformanceBridge Practice in all 28 of its radiology departments in a two-phased approach, starting with their 16 Arizona and academic sites, then expanding to its 12 radiology departments in five other states.
Philips recently announced that it acquired VitalHealth, a provider of cloud-based population health management solutions for the delivery of personalized care outside of the hospital, for example, in regional care networks. Headquartered in the Netherlands, VitalHealth’s products and services are being used by more than 100 healthcare networks in various countries including the United States, India, China, Sweden, Germany, Belgium and the Netherlands. The company was founded in 2006 by Mayo Clinic (U.S.) and Noaber Foundation (the Netherlands) and employs approximately 200 employees. Financial details of the transaction were not disclosed.
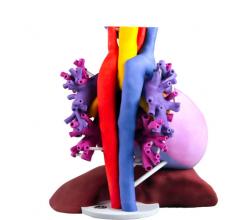
Philips recently announced agreements with 3D Systems and Stratasys, two global leaders in the 3-D printing industry, to help progress patient care and improve the clinician experience. Advanced 3-D modeling provides radiologists with additional views to help strengthen anatomical knowledge which could enhance clinical impact in reviewing complex, multi-disciplinary cases.
Philips showcased the company’s latest magnetic resonance (MR) imaging solutions at the Radiological Society of North America’s 2017 annual meeting, Nov. 26-Dec. 1 in Chicago. This includes their newest MR system, MR Prodiva 1.5T (which is still U.S. Food and Drug Administration [FDA] 510(k) pending) that provides enhanced clinical performance, workflow and patient experience. Philips also unveiled new solutions to drastically reduce exam times and elevate neuro-oncology.
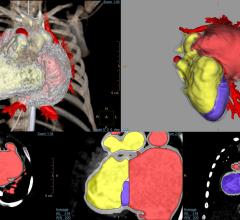
Philips announced the debut of IntelliSpace Portal 10, the latest edition of its comprehensive, advanced visualization and quantification platform, at the 2017 Radiological Society of North America (RSNA) Annual Meeting, Nov. 26-Dec. 1 in Chicago. This next generation features enhancements across the solution, with a particular focus on oncology to provide radiologists with an improved full set of applications and workflows to support the reading and follow-up of complex oncology cases. IntelliSpace Portal 10 also includes a new 3-D modeling application and has been expanded with the DynaCAD Prostate and Breast solutions through integration with InVivo.


 March 06, 2018
March 06, 2018