If you enjoy this content, please share it with a colleague
Blue Earth Diagnostics Ltd.
RELATED CONTENT
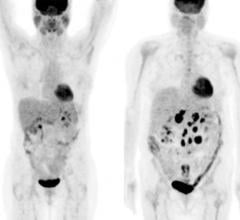
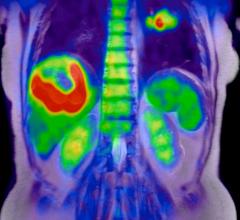
Blue Earth Diagnostics Ltd. announced the peer-reviewed publication of results from a Phase 3 clinical trial of Axumin (fluciclovine F 18) injection. Axumin is a novel molecular imaging agent indicated for use in positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood levels of prostate-specific antigen (PSA) following prior treatment. Results of the multi-center study, conducted in Norway, Italy and the United States, demonstrated a 68 percent overall detection rate (DR) for Axumin, with the ability to detect local, as well as distant, prostate cancer recurrence across a wide range of PSA values. Axumin was well-tolerated in the study.
September 7, 2016 — Blue Earth Diagnostics announced that the first post-U.S. Food and Drug Administration (FDA) ...
June 7, 2016 — Blue Earth Diagnostics Ltd. and Siemens’ PETNET Solutions Inc. announced the commercial availability of ...
Blue Earth Diagnostics, a molecular imaging diagnostics company, announced it has established operations in the United States in Burlington, Mass.
Blue Earth Diagnostics Ltd (BED) announced it has received both U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) Orphan Drug Designation (ODD) for fluciclovine (18F) in the diagnosis of glioma. Fluciclovine is an investigational positron emission tomography (PET) radiopharmaceutical based on a synthetic amino acid, which appears to be preferentially taken up in a number of cancer indications, including in prostate cancer and brain tumors.
Siemens’ PETNET Solutions Inc. has entered into a nationwide agreement with U.K.-based Blue Earth Diagnostics Ltd. for the exclusive commercial manufacturing and distribution of Fluciclovine (18F), an investigational positron emission tomography and computed tomography (PET/CT) radiopharmaceutical. Fluciclovine (18F) is being studied for prostate imaging in clinical trials conducted in the United States, Japan, Italy, Norway, Sweden and Finland. The F-18-based radiopharmaceutical has a long half-life, which may facilitate its geographical distribution to clinical trial sites and then to clinical imaging centers once it gains approval from the U.S. Food and Drug Administration (FDA).

 October 25, 2016
October 25, 2016