If you enjoy this content, please share it with a colleague
Blue Earth Diagnostics Ltd.
RELATED CONTENT
September 17, 2021 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and ...
May 25, 2021 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization ...
January 18, 2021 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and ...
November 25, 2020 — Blue Earth Diagnostics, a leading molecular imaging diagnostics company, today announced that their ...
Bracco Imaging S.p.A. has signed a definitive agreement to acquire Blue Earth Diagnostics, a molecular imaging company based in Oxford, U.K. Subject to customary closing conditions, completion of the transaction is expected in the third quarter of this calendar year.
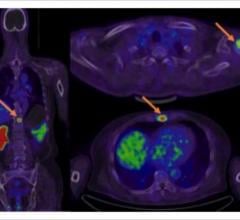
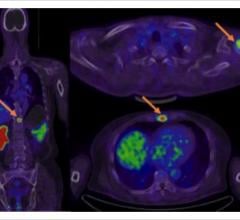
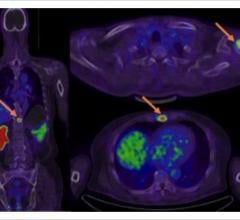
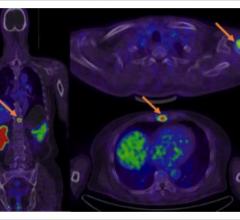
Blue Earth Diagnostics announced expanded access to the Axumin (fluciclovine (18F)) imaging agent in Europe. The first commercial production of Axumin in Belgium occurred recently, with the first Belgian patients being dosed. This follows the first administration of Axumin in Luxembourg earlier this year. Axumin is the first and only positron emission tomography (PET) imaging agent approved in the European Union for use in men with suspected recurrent prostate cancer. Axumin is commercially available in Belgium, Luxembourg, Italy, France, Norway, the Czech Republic, The Netherlands, United Kingdom and Austria, with further European countries set to follow soon.
Blue Earth Diagnostics signed an exclusive, worldwide agreement with Scintomics GmbH, Germany, a specialist in radiopharmaceuticals and radiopharmaceutical technologies. Under the terms of the agreement, Blue Earth Diagnostics has acquired the exclusive worldwide rights to a broad family of prostate specific membrane antigen (PSMA)-targeted radiohybrid (“rh”) agents for cancer imaging, together with an exclusive option to explore therapeutic applications.
Blue Earth Diagnostics announced that Axumin (fluciclovine F 18) injection has been added to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Prostate Cancer (Version 1.2018). These updated NCCN Guidelines state that F-18 fluciclovine positron emission tomography/computed tomography (PET/CT) or PET/magnetic resonance imaging (MRI) should be considered in the clinical workup of patients with recurrence or progression of their prostate cancer. This recommendation is based on uniform NCCN consensus that the intervention is appropriate.
Blue Earth Diagnostics announced that the Trial Steering Committee recommended further recruitment be stopped in the FALCON clinical study of fluciclovine (18F) PET/CT imaging, based on successful results of a pre-planned interim analysis. The FALCON trial, announced in March 2016, is a U.K.-based, open-label study (NCT02578940) to evaluate the clinical impact of fluciclovine (18F) positron emission tomography/computed tomography (PET/CT) imaging on patient management decisions in men with biochemically recurrent prostate cancer.
Blue Earth Diagnostics and Siemens’ PETNET Solutions announced that an increasing number of radiopharmacies will offer Blue Earth Diagnostics’ Axumin (fluciclovine F-18) positron emission tomography (PET) imaging agent through PETNET’s national network.


 September 17, 2021
September 17, 2021