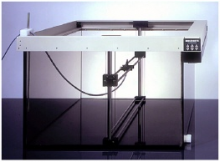
Swissray received U.S. Food and Drug Administration (FDA) approval earlier in 2016 for the ddRAura OTC digital X-ray systems, including the ddrAura OTC with Automated Positioning System (APS).
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now