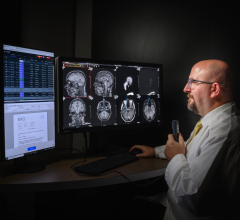
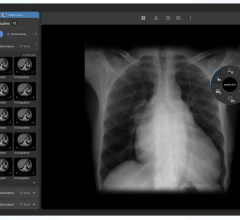
January 13, 2015 — Viztek announced the U.S. Food and Drug Administration (FDA) approval of its Exa PACS. Completely web-based and compatible with all devices and operating systems, the Exa PACS delivers efficiency and productivity benefits to hospitals, imaging centers and teleradiology groups via PACS accessibility and utility for end users.
Exa's zero footprint technology allows the viewer to be fully operational within a web browser, eliminating the requirement to install additional programs that traditional viewers need. Viztek was the first PACS provider to license the zero footprint technology. Server-side rendering allows for physicians to bypass the delays of prefetching or auto-routing to instantaneously open large studies, which enables a faster, streamlined workflow and provides physicians with immediate access from any location with full diagnostic quality viewing and advanced utility, with nearly any level of Internet connection.
While the diagnostic benefits are key for radiologists, Exa also provides significant benefits for the IT department. In hospital settings, where radiology departments commonly rely on 1000-slice CT, digital mammography and other modalities generating significant files sizes of 600 MB and larger, the relief provided via Exa is substantial and cost-effective. The server-side rendering of the PACS eliminates the need for radiology departments to transfer large data sets around, significantly reducing strain on the greater hospital network while alleviating organizations from the costs of needing to bolster their networks.
The Viztek Exa PACS works in tandem with the Exa EHR, which has bundled all essential components of the radiology workflow process including PACS, practice management, billing, order entry, radiology reports, patient portals and other software product currently delivered by the company.
For more information: www.viztek.net


 November 29, 2025
November 29, 2025