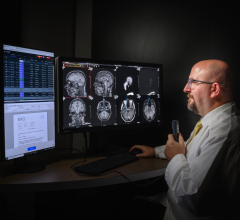
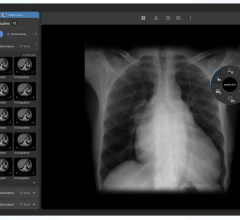
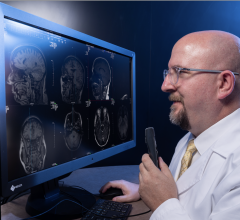
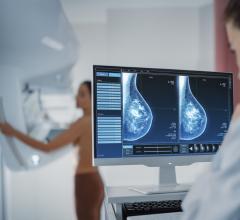
January 19, 2016 — RamSoft announced in December its ISO 13485:2003 certification, a quality standard that certifies compliance to the Canadian Medical Devices Conformity Assessment System (CMDCAS), the FDA’s Quality System Requirements, and the European Union Medical Device Directive. This achievement applies to the company’s full suite of picture archiving and communication systems (PACS), radiology information systems (RIS)/PACS, teleradiology and patient information management systems.
RamSoft’s quality management system (QMS) is driven by a quality policy and quality objectives. Through it, RamSoft works to increase client satisfaction, product efficiency, and internal operations by effectively collecting, analyzing and responding to feedback.
For more information: www.ramsoft.com


 July 25, 2024
July 25, 2024