November 19, 2018 – QView Medical will showcase QVCAD, the first U.S. Food and Drug Administration (FDA)-approved artificial intelligence (AI) computer-aided detection (CAD) system for concurrent reading of automated breast ultrasound (ABUS) exams, at the 104th Annual Radiological Society of North America (RSNA) meeting, Nov. 25-30, in Chicago. Being demonstrated for the first time since being approved, QVCAD reduces interpretation time of screening ABUS exams while maintaining diagnostic accuracy.
In addition, QView successfully completed an installation of QVCAD at Northern Arizona Healthcare, the first joint installation with the Invenia ABUS system from GE Healthcare. Invenia ABUS is approved by the FDA for breast cancer screening as an adjunct to mammography for asymptomatic women with dense breasts. “We are excited to add the capability to offer ABUS as part of our comprehensive breast cancer screening program, which will help improve cancer detection for women with dense breasts. However, it was important that we implement with concurrent decision support from QVCAD to improve reading times while maintaining workflow and diagnostic confidence,” said Liz Palomino, MHA, RT, Northern Arizona Healthcare director of medical imaging, Flagstaff Medical Center, Sedona Medical Center, Verde Valley Medical Center and Verde Valley Medical Imaging Center.
Results from several clinical studies have shown that the addition of ABUS to screening mammography results in a significant increase in cancer detection in women with dense breasts. However, the interpretation of ABUS exams, with up to 2,000 images per case, is complex and time consuming, particularly for new users. According to results of a reader study published recently in the American Journal of Radiology, QVCAD reduced reader interpretation time by 33 percent while maintaining diagnostic accuracy.
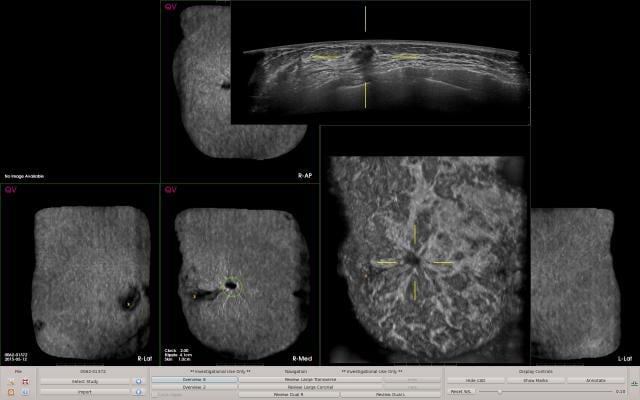
Based on deep learning algorithms, the AI system is designed to detect suspicious areas of breast tissue with characteristics similar to breast lesions, and highlight suspicious area to distinguish potentially malignant lesions from normal breast tissue. QVCAD is FDA-approved for use with ABUS systems and has received the CE mark for use with ABUS/ABVS systems.
To improve reader productivity, QVCAD provides synthetic 2-D images of all six volumetric datasets in a standard ABUS exam to provide an immediately visual overview of the case. The C-thru images, which are minimum intensity projections (MinIP), summarize each 3-D ABUS volume in a 2-D image. They bring attention to specific areas of interest by enhancement of radial spiculations and retraction patterns in coronal reconstructions, which are highly suggestive of breast cancer in ABUS. Users may select any CAD mark or area of interest on the C-thru image and the corresponding original ABUS images will be displayed, enabling users to efficiently review the entire ABUS case.
For more information: www.qviewmedical.com


 March 19, 2025
March 19, 2025 







