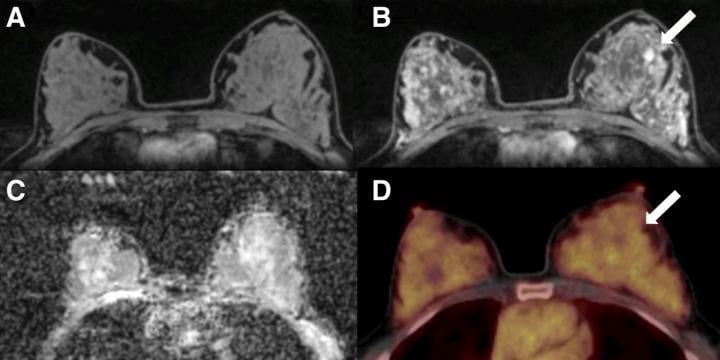
A 50-y-old postmenopausal woman with fibroadenoma (arrows) in left breast. (A) Unenhanced fat-saturated T1-weighted MRI shows extreme amount of FGT (ACR d). (B) Moderate BPE is seen on dynamic contrast-enhanced MRI at 90 s. (C) Mean ADC of breast parenchyma of contralateral breast on diffusion-weighted imaging with ADC mapping is 1.5 × 10?3 mm2/s. (D) On 18F-FDG PET/CT, lesion is not 18F-FDG-avid, and BPU of normal breast parenchyma is relatively high, with SUVmax of 3.2. Photo courtesy of K Pinker, et al., Medical University of Vienna, Vienna, Austria
January 27, 2020 — Researchers have identified several potentially useful breast cancer biomarkers that indicate the presence and risk of malignancy, according to new research published in the January issue of The Journal of Nuclear Medicine. By comparing healthy contralateral breast tissue of patients with malignant breast tumors and benign breast tumors, researchers found that multiple differences in biomarkers can be assessed with PET/MRI imaging, which could impact risk-adapted screening and risk-reduction strategies in clinical practice.
In breast cancer, early detection remains key to improved prognosis and survival. While screening mammography has decreased mortality for breast cancer patients by 30 percent, its sensitivity is limited and is decreased in women with dense breast tissue. "Such shortcomings warrant further refinements in breast cancer screening modalities and the identification of imaging biomarkers to guide follow-up care for breast cancer patients," said Doris Leithner, M.D., research fellow at Memorial Sloan Kettering Cancer Center in New York, N.Y. "Our study aimed to assess the differences in 18F-FDG PET/MRI biomarkers in healthy contralateral breast tissue among patients with malignant or benign breast tumors."
The study included 141 patients with imaging abnormalities on mammography or sonography on a tumor-free contralateral breast. The patients underwent combined PET/MRI of the breast with dynamic contrast-enhanced MRI, diffusion-weighted imaging (DWI) and the radiotracer 18F-FDG. In all patients, several imaging biomarkers were recorded in the tumor-free breast: background parenchymal enhancement and fibroglandular tissue (from MRI), mean apparent diffusion coefficient (from DWI) and breast parenchymal uptake (from 18F-FDG PET). Differences among the biomarkers were analyzed by two independent readers.
A total of 100 malignant and 41 benign lesions were assessed. In the contralateral breast tissue, background parenchymal enhancement and breast parenchymal uptake were decreased and differed significantly between patients with benign and malignant lesions. The difference in fibroglandular tissue approached but did not reach significance, and the mean apparent diffusion coefficient did not differ between the groups.
"Based on these results, tracer uptake of normal breast parenchyma in 18F-FDG PET might serve as another important, easily quantifiable imaging biomarker in breast cancer, similar to breast density in mammography and background parenchymal enhancement in MRI," Leithner explained. "As hybrid PET/MRI scanners are increasingly being used in clinical practice, they can simultaneously assess and monitor multiple imaging biomarkers--including breast parenchymal uptake--which could consequently contribute to risk-adapted screening and guide risk-reduction strategies."
For more information: www.snmmi.org


 March 10, 2025
March 10, 2025