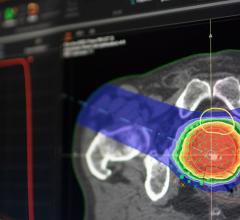
December 23, 2008 - The FDA cleared Ion Beam Applications’ (IBA) cyclotron-based integrated commercial proton therapy system with pencil beam scanning (PBS), a delivery method that allows for precise dose delivery by its proton therapy system.
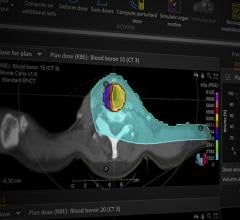
PBS technology was developed by IBA in close collaboration with the staff of Massachusetts General Hospital (MGH) at the Francis H. Burr Proton Therapy Center in Boston. The PBS delivery method is characterized by the use of a proton beam that is actively scanned throughout the target tumor volume. During a PBS treatment, the transverse beam position, longitudinal beam position (range) and dose are precisely controlled and adjusted to deliver the prescribed dose in the target. Compared to conventional passive scattering techniques used to treat cancer with protons, PBS is designed to better 3D conformity to the target, in order to allow for improved sparing of organs at risk and healthy tissues.
IBA is the first company with a cyclotron-based proton therapy system to receive approval from the FDA for Pencil Beam Scanning. In addition to PBS, universal nozzle provides uniform scanning, double and single scattering delivery methods, offering to the proton therapy practitioners the flexibility to select the preferred beam delivery method.
For more information: www.iba-worldwide.com

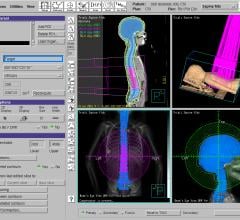
 May 06, 2024
May 06, 2024