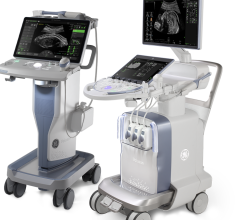
November 19, 2015 — Fujifilm SonoSite Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for iViz, its newest ultrasound medical visualization solution. First commercialized in Europe, iViz was developed from the ground up to meet the needs of the highly-mobile clinician.
Clinicians do not always treat patients in traditional care settings or have easy access to supporting hospital infrastructure; instead they rely on tools that can easily be carried to help diagnose a patient’s condition. With iViz, clinicians can seamlessly access learning resources and patient information, store exam findings, submit reports and consult with remote providers for near real-time assessment, making it especially suited for field use and for the growing area of telemedicine.
According to the company, iViz is the first medical visualization solution that is enabled for bi-directional electronic medical record (EMR) connectivity through the Synapse VNA (vendor neutral archive). Using this option, iViz accepts patient demographics from the EMR, eliminating manual entry and saving valuable time. With just a few taps, iViz can also send patient reports to the EMR. Based on the Android operating system, the iViz platform will include Web browsing, email and electrocardiogram (ECG) capability.
The system is designed to be highly accessible and lightweight. It features a high-resolution, 7-inch display touchscreen, a wide dynamic range and vibrant color flow images, as well as imaging modes and exams to meet the clinical needs of different medical providers.
For more information: www.sonosite.com


 July 30, 2024
July 30, 2024