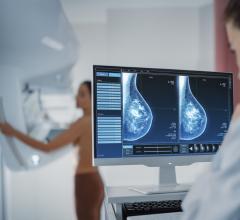
Aspyra adds mammography viewing to AccessNET PACS.
March 24, 2010 - The FDA 510(k) has issued marketing clearance for a PACS (picture archive and communication system), which has added viewing capabilities for digital mammography studies for diagnostic interpretation using FDA approved monitors.
The new PACS, Aspyra AccessNET PACS, will now include the display of mammography CAD results, the IHE conformant hanging protocols, and other display capabilities for digital mammography studies.
The system integrates with the on-board functionality of the Matrox Xenia card engineered to provide performance and quality improvements using high resolution displays and images required for mammography.
Other recent enhancements to the PACS target performance improvement for accessing and reading radiological images. These include compression for transferring images and dictations from remote locations, improvements in the navigation and functionality of commonly used imaging tools, and support for management of critical results.
For more information: www.aspyra.com


 February 03, 2025
February 03, 2025