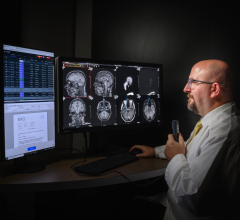
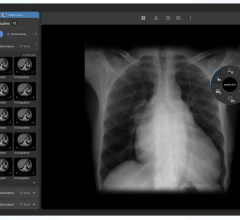
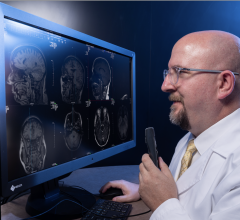
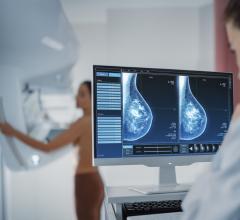
The FDA gave market approval to the Medical Office Suite of Products, which aims to address the issue of coordination between clinical modality applications, PACS systems, information systems and reporting packages.
The reporting component is designed to provide the latest technology within the DICOM Structured Reporting standard and proper reports along with their correlative worksheets are auto-populated with the measured and derived data gathered during the exam. A full repertoire of DICOM tools, such as modality worklist, can be included with the application, which will in-turn auto-populate the patient information directly into the modality equipment. The Medical Office also provides production-based tools to support report publishing and more.


 July 25, 2024
July 25, 2024