July 8, 2015 - The first randomized trial investigating the additional value of magnetic resonance imaging (MRI) for screening women with dense breasts, is featured in the current issue of Radiology. The article, "MR Imaging as an Additional Screening Modality for the Detection of Breast Cancer in Women Aged 50–75 Years with Extremely Dense Breasts: The DENSE Trial Study Design," presents the rationale and design of the DENSE Trial. Run by Carla van Gils, M.D., and Wouter Veldhuis, M.D., from University Medical Center Utrecht (UMCU) in the Netherlands, the trial seeks to determine the effectiveness of screening with mammography and MRI compared to mammography alone in women who have extremely dense breasts.
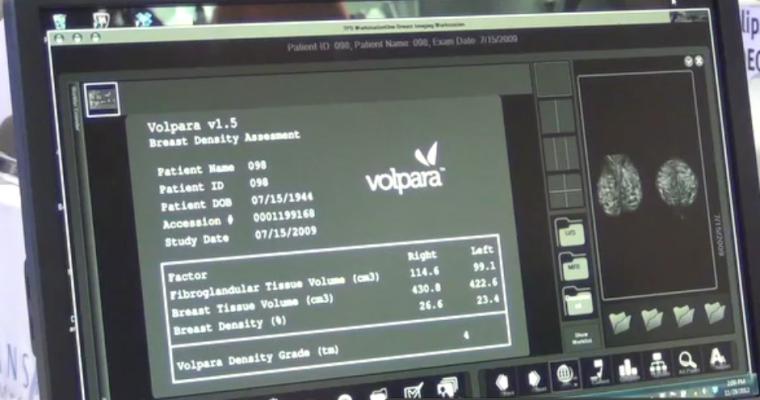
Approximately 1 million women are screened every year in the Netherlands as part of the Dutch Breast Screening Program. To study the additional value of MRI, the DENSE Trial uses a randomized controlled design, with one group receiving mammography and the other group receiving mammography and MRI. VolparaDensity software from Volpara Solutions is being used in the trial to provide objective, volumetric breast density values. Participants with extremely dense breasts and a negative mammography result are randomized into two arms: one to have an additional MRI (n=7,237) and the other to follow the usual mammography screening program (n=28,948). The primary outcome is the difference in proportion of interval cancers between the arms. In order to be an effective screening strategy, the extra MRI screen-detected cancers have to be accompanied by a subsequent reduction in interval cancers.
Research has shown that mammography alone has significantly lower sensitivity in women with extremely dense breasts than in women with fatty breasts. With high sensitivity, even in the dense breast, MRI has the potential to improve cancer detection at an early stage; however, MRI is more costly and can increase the number of false-positives. The DENSE Trial aims to validate personalization of the national breast cancer screening program by incorporating information on mammographic density.
"Automated, objective density assessment was critical for this project, which is why we selected Volpara. The software's robust nature and clinical track record was once again validated by the results of the ASSURE project we recently presented at ECR, which demonstrated the direct correlation of mammographic screening performance to each patient's breast density categorization as determined by VolparaDensity," stated van Gils.
"The major strength of the DENSE Trial is its parallel-group randomized controlled design. As far as we are aware, DENSE is the first randomized trial investigating the additional value of MRI in women with extremely dense breasts" she added.
VolparaDensity is in use at breast imaging centers worldwide to help radiologists objectively assess density from both digital mammography and tomosynthesis images, and to determine which women would benefit from additional screening. Highly correlated to breast MR assessments, VolparaDensity automatically generates an objective measurement of volumetric breast density correlated to the American College of Radiology (ACR) breast density categories. To date, more than 5 million women have had their breast density analyzed using VolparaDensity.
For more information: www.volparasolutions.com


 March 10, 2025
March 10, 2025