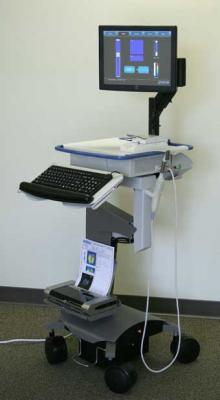
August 12, 2009 - The second of three centers in ProUroCare Medical's FDA clinical study of the ProUroScan imaging system has completed its portion of the study.
The ProUroScan imaging system is designed for use as an aid to the physician in visualizing and documenting tissue abnormalities in the prostate that have been previously detected by a digital rectal exam ("DRE"). As an adjunct to DRE, the ProUroScan system will be used following an abnormal DRE to generate a real time image and map of the prostate and to store this information electronically.
ProUroCare began the current study with the objective of enrolling at least 10 patients in three sites and having more than 40 patients enrolled overall. Since beginning, five clinical sites have been established, and as of last week, 44 patients have already been enrolled. The last of the three clinical studies to have 10 patients or more enrolled has only four patients remaining to meet their study objectives. ProUroCare expects to have 50 or more patients evaluated for their FDA submission, which is planned for fourth quarter 2009. Although the company is not in a position to discuss specifics of the results achieved to date, Rick Carlson, CEO of ProUroCare stated that, "the initial results are consistent with earlier reported results and those that were expected for this study. We are one step closer to the possibility of providing patients with an image of their prostate that can be stored and subsequently reviewed."
In addition to the current clinical trial, a large study involving 168 patients was conducted by Artann Labs beginning in November of 2004. The results of this early work were presented in an article published in Urology, a peer reviewed journal, in March of 2008. The article states that in 84 percent of the cases, the imaging system was able to reconstruct and store a cross section image of the prostate.
For more information: www.prourocare.com


 August 06, 2024
August 06, 2024 








