If you enjoy this content, please share it with a colleague
BTG plc
RELATED CONTENT
Boston Scientific Corp. announced the completion of its acquisition of BTG plc. pursuant to the previously announced scheme of arrangement. BTG develops and commercializes products used in minimally invasive procedures targeting cancer and vascular diseases, as well as specialty pharmaceuticals.
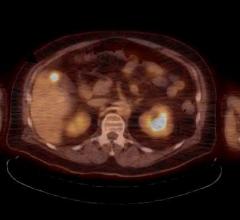
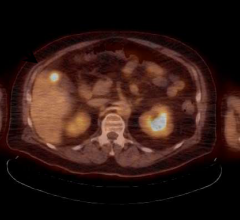
New consensus recommendations for personalized treatment for hepatocellular carcinoma (HCC) with BTG’s TheraSphere have been published by an international multidisciplinary working group1. In addition, data from the Dosisphere phase II dosimetry trial supports MAA single photon emission computed tomography (SPECT)/CT-based prospective personalized dosimetry2.
British medical device maker BTG PLC and its subsidiary, Biocompatibles Inc., have agreed to pay $36 million to the U.S. government to resolve federal and state False Claims Act (FCA) allegations resulting from the off-label promotion of LC Bead, a medical device approved for use only for embolization of hypervascular tumors. $25 million of the settlement is to resolve the civil qui tam action, which alleged fraud, and $11 million of the settlement is a criminal fine against Biocompatibles.
BTG plc and Royal Philips announced a significant milestone in their collaboration with the treatment of the first liver cancer patient with LC Bead LUMI in conjunction with Philips Live Image Guidance. The treatment targeted a hypervascular tumor with the goal to block blood flow to achieve tumor necrosis.
Mirada Medical Ltd, a medical imaging software company, and BTG plc, a global specialist healthcare company, announced a collaboration to develop dosimetry software solutions to optimize radioembolization therapy with TheraSphere.


 August 20, 2019
August 20, 2019