If you enjoy this content, please share it with a colleague
Brainlab
RELATED CONTENT
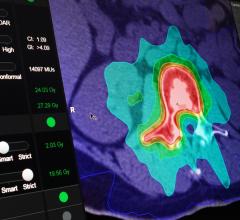
Brainlab announced the implementation of a software solution that can achieve better radiation dose conformity around brain tumors, resulting from a close strategic collaboration with Elekta. Brainlab Elements take advantage of the specific properties of Elekta’s Agility multileaf collimator and compensates for the width of selected leaves, enabling the radiation beam to more accurately conform to the tumor’s shape, thereby minimizing dose to organs at risk.
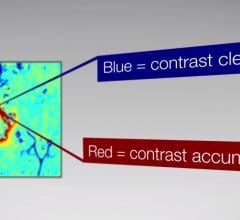
Brainlab announced U.S. Food and Drug Administration (FDA) clearance of its Contrast Clearance Analysis methodology, developed at Sheba Medical Center in Tel-Hashomer, Israel, with technology provided by Brainlab. The software analyzes magnetic resonance (MR) images to differentiate regions of efficient contrast clearance from regions with contrast accumulation in most cranial tumor patients to provide additional insight into post-treatment tumor characteristics.
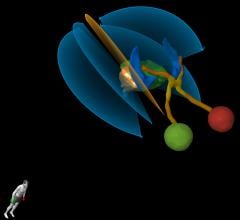
Elekta and Brainlab have reconfirmed their alliance for the integrated use of the Elekta Versa HD linear accelerator and Brainlab ExacTrac patient positioning and monitoring technology. The integration of these two technologies offers high-definition stereotactic radiosurgery (HDRS) treatments with versatile patient positioning and monitoring through simplified workflows. A Versa HD system augmented with ExacTrac was displayed at the Elekta booth during the American Society for Radiation Oncology (ASTRO) Annual Meeting, Sept. 24-27 in San Diego.
Brainlab announced that it has received U.S. Food and Drug Administration (FDA) clearance for Elements Spine SRS and Elements Cranial SRS, two software applications for patient-tailored planning of radiosurgery treatments for indications of the spine and brain. With FDA clearance of these software modules, the Brainlab Elements portfolio of applications that facilitate quick planning, spare organs at risk, and create highly conformal radiation doses in the brain and spine is complete and ready for clinical use in the United States.
July 31, 2016 — Brainlab is showcasing innovations for the complete radiosurgery process, including arc trajectory ...
Brainlab showcased its new Elements Cranial SRS (stereotactic radiosurgery) software at this year’s European Society for Radiation Oncology (ESTRO) 35, April 29-May 3 in Turin, Italy.
Brainlab announced that it has enrolled 11 of the expected 30 hospitals and healthcare systems in the national Stereotactic Radiosurgery (SRS) Patient Registry.
Brainlab AG and Dalhousie University, Atlantic Canada’s leading research-intensive university, have entered into an exclusive intellectual property license agreement for integration with next-generation radiosurgery software.
On Feb. 19-20, Brainlab sponsored the Sixth International Conference of the Novalis Circle in Munich, Germany, bringing together international experts in radiation oncology and neurosurgery.
Brainlab announced the results of a study with leading international cancer centers aimed at understanding the safety and efficacy of stereotactic body radiation therapy (SBRT) in the treatment of patients with prostate cancer.

 April 24, 2018
April 24, 2018