If you enjoy this content, please share it with a colleague
RELATED CONTENT
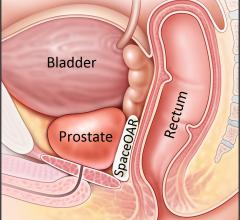
Boston Scientific Corp. announced the close of its acquisition of Augmenix Inc., developer of the SpaceOAR Hydrogel System to help reduce common and debilitating side effects that men may experience after receiving radiotherapy to treat prostate cancer. The biodegradable SpaceOAR hydrogel is injected between the rectum and prostate to decrease a patient's exposure to rectal radiation and thereby reduce rectal radiation injury – one of the most common complications of prostate radiotherapy.
Boston Scientific has entered into a definitive agreement to acquire Augmenix Inc., a privately-held company which has developed and commercialized the SpaceOAR System to reduce common and debilitating side effects that men may experience after receiving prostate cancer radiotherapy. The transaction consists of an upfront cash payment of $500 million, and up to $100 million for reaching sales-based milestones.
Augmenix K.K. announced that SpaceOAR hydrogel, a soft, implanted absorbable gel spacer is now available to all prostate cancer radiotherapy patients in Japan through the Ministry of Health, Labour & Welfare (MHLW) national reimbursement. SpaceOAR hydrogel is clinically proven to assist in the decrease of unwanted side effects from radiotherapy treatment in patients with prostate cancer.
A multicenter clinical trial being led by UT Southwestern physicians is testing a technique for sparing nerve bundles and arteries involved in sexual function to preserve potency in patients getting radiation therapy for prostate cancer.
September 22, 2017 — Augmenix Inc. announced a preview of clinical data that will be presented at the 59th Annual ...
Augmenix Inc. announced that the National Institute for Health and Care Excellence (NICE) in the U.K. has issued Interventional Procedure Guidance (IPG) supporting the use of a hydrogel barrier to reduce the risk of toxicity to surrounding tissue in radiotherapy to treat prostate cancer. The guidance recommends use of hydrogel spacers under "standard arrangements". For a procedure to be recommended for use with standard arrangements (previously called “normal arrangements”), the evidence should be adequate to show safety and efficacy of the procedure both in the long term and short term. NICE’s guidance clearly indicates that this procedure meets the highest evidence standards.
Augmenix Inc. announced that Palmetto GBA LLC — the Medicare Administrative Contractor (MAC) covering North Carolina, South Carolina, Virginia and West Virginia — will establish coverage for SpaceOAR hydrogel when medically necessary, effective Oct. 2, 2017. The change will enable Medicare beneficiaries in these states access to SpaceOAR hydrogel for use in prostate cancer radiotherapy.
Augmenix Inc. announced that the first patient in New Zealand has been treated with SpaceOAR hydrogel at the Kathleen Kilgour Centre (KKC) in Tauranga. SpaceOAR hydrogel is the first absorbable spacer designed to separate the rectum and prostate to reduce the risk of long-term side effects after radiation treatment.
January 16, 2017 — Augmenix Inc. announced that the International Journal of Radiation Oncology Biology and Physics ...
Augmenix Inc. announced that the American Medical Association (AMA) has granted a Category I, Current Procedural Terminology (CPT) code specifically for periprostatic implantation of biodegradable material. AMA granted the code with the support of the American Society for Radiation Oncology (ASTRO) and the American Urological Association (AUA).


 October 17, 2018
October 17, 2018