September 5, 2017 — Augmenix Inc. announced that Palmetto GBA LLC — the Medicare Administrative Contractor (MAC) covering North Carolina, South Carolina, Virginia and West Virginia — will establish coverage for SpaceOAR hydrogel when medically necessary, effective Oct. 2, 2017. The change will enable Medicare beneficiaries in these states access to SpaceOAR hydrogel for use in prostate cancer radiotherapy.
Palmetto posted a revision to its Local Coverage Determination (LCD) Non-Covered Category III CPT Codes (L34555) deleting American Medical Association (AMA) CPT code 0438T, Transperineal placement of biodegradable material, indicating that this is now a covered procedure effective 10/02/2017. Currently, AMA CPT code 0438T is typically the AMA CPT code used to report the SpaceOAR hydrogel procedure.
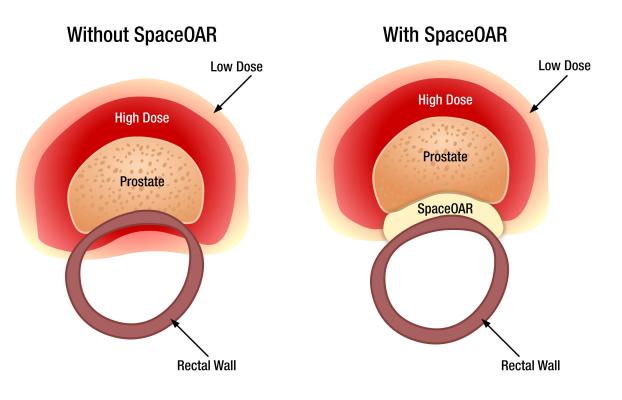
SpaceOAR hydrogel reduces rectal injury in men receiving prostate cancer radiation therapy by acting as a spacer – pushing the rectum away from the prostate. This space between organs decreases the radiation dose to the rectum and other organs at risk (OAR). Earlier this year, Augmenix announced published data from their prospective, randomized clinical trial showing that patients treated with SpaceOAR hydrogel prior to prostate cancer radiotherapy demonstrated significant rectal (bowel), urinary and sexual benefit through three years of follow-up.
In November 2016, the AMA created a new Category I CPT Code for the SpaceOAR hydrogel procedure that will become effective on Jan. 1, 2018. The new code will replace AMA CPT code 0438T.
For more information: www.augmenix.com


 April 21, 2025
April 21, 2025