The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, a high-definition CT scanner that is said to produce images 100 ...
June 9, 2008 - Cardinal Health today launched a new system that leverages the company’s dispensing technologies to ...
The Codonics Virtua Medical Disc Publisher is designed to set a new standard for speed, efficiency and ease of use in ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
June 9, 2008 - Siemens Medical Solutions gained FDA 510(k) market clearance for the SOMATOM Definition AS, what is said ...
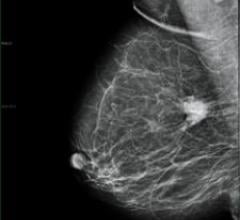
iCAD’s SecondLook Digital Computer-Aided Detection system for mammography received FDA clearance for sale with FUJIFILM ...

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Bayer HealthCare Pharmaceuticals collaborated with Mobile Aspects to create VistaTrak, a touchscreen contrast media ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
June 9, 2008 - Curlin Medical was recently awarded a three-year contract to provide its advanced pain management-local ...
Viztek introduced fully equipped mobile imaging vehicles, which includes a Honda Element equipped with Viztek Opal-RAD PACS and Kodak Point-of-Care CR, providing radiology groups with a turnkey solution to broaden their patient base, also making diagnostic imaging accessible in impoverished areas.
June 9, 2008 - The FDA cleared Mindray’s DC-3 color ultrasound imaging system, equipped with extensive applications in ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
With radiologists in short supply, the need for teleradiology services continues to grow and the market has responded in ...
Candelis received 510(k) marketing clearance for its ImageGrid Mammography Web Viewer and ImageGrid Radiology Web Viewer ...
June 9, 2008 – Electronic health records maker MediNotes Corp. said it added 199 new software agreements with new and ...
Having the most efficient clinical workflows with enhanced diagnostic capabilities is a major goal for clinicians and ...
Acusphere has submitted a New Drug Application (NDA) to the FDA for approval to market Imagify (Perflubutane Polymer Microspheres for Injectable Suspension), an ultrasound imaging agent for the detection of coronary artery disease, which could prove as accurate as nuclear stress testing.
June 9, 2008 - Hologic Inc. said today that it has signed a definitive agreement to acquire Third Wave Technologies Inc ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
June 9, 2008 - At the request of its membership, the American College of Physicians (ACP) recently conducted an ...
Toshiba Teli America introduced the T24MSA001-MD medical-grade 24-inch LCD color widescreen monitor with full HD (1,080 ...
ProHance is a macrocyclic, nonionic, gadolinium-based magnetic resonance imaging (MRI) contrast agent designed for use in MRI in adults and children over two years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues. ProHance is also indicated for use in MRI in adults to visualize lesions in the head and neck.
June 9, 2008 - The administration of imprecise dosages, particularly to small children, remains a serious problem faced ...
The FDA granted tentative approval for Covidien’s Abbreviated New Drug Application (ANDA) for its Kit for the Preparation of Technetium Tc 99m Sestamibi Injection. Covidien’s tentatively approved product is a generic of Cardiolite, which is a myocardial perfusion imaging agent used for detecting coronary artery disease.
June 9, 2008 - Siemens unveiled the ACUSON SC2000 volume imaging ultrasound system, which acquires nonstitched, real ...


 June 08, 2008
June 08, 2008