September 28, 2011 – Researchers have a new weapon in their arsenal to diagnose and treat traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) among military service members and civilians. The National Institutes of Health Clinical Center began imaging patients last week on a first-of-its-kind, whole-body simultaneous positron emission tomography (PET) and magnetic resonance imaging (MRI) device. The Biograph mMR offers a more complete picture of abnormal metabolic activity in a shorter time frame than separate MRI and PET scans, two tests many patients undergo.
September 28, 2011 – Hie Electronics, a manufacturer of long-term scalable Active Archive data storage solutions and the manufacturer of the TeraStack Solution, announced that it has received a registration with the U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health.
September 28, 2011 – Bionix Radiation Therapy announces the addition of Breath Hold, an interactive breath-hold control monitoring system from MedSpira, as an accessory to its Omni V Patient Positioning System. Developed by Mayo Clinic physicians, Breath Hold is a user-friendly system that enables patients to easily and consistently reproduce a breath-hold reference point consistent breathing pattern. This consistency is a significant benefit, ideal for stereotactic radiation therapy procedures involving the lungs and upper abdomen.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
September 28, 2011 – Calypso Medical Technologies Inc., developer of real-time localization technology used for precise tumor tracking, announced that the U.S. Food and Drug Administration (FDA) granted investigational device exemption (IDE) approval for its clinical study evaluating real-time tracking of lung cancer tumors during radiation delivery. Patient enrollment is planned at Washington University in St. Louis, and the Cancer Treatment Centers of America in Tulsa, Oklahoma. Investigators in the United States will implant three anchored Beacon transponders in patients’ lungs and use the Calypso System to precisely track tumor location and movement during lung cancer radiation therapy.
September 28, 2011 –Ziehm Imaging said its holding company Aton GmbH acquired 100 percent of the United States-based mini C-arm specialist OrthoScan Inc.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
September 28, 2011 – At ASTRO, Civco Medical Solutions will highlight the Protura Robotic Patient Positioning System and multiple motion management solutions, including the RFSuite RFID Tracking & Verification System.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
September 28, 2011 – The U.S. Food and Drug Administration (FDA) has cleared two Hologic Sentinelle magnetic resonance imaging (MRI) Solutions products: Sentinelle Endo Coil Array for pelvic imaging including the prostate, cervix, colon and the surrounding tissues in the pelvis, and Sentinelle’s 16 -channel Breast Coil Array for use with Siemens 1.5T and 3.0T MRI systems. Both coils will be featured at Radiology Society of North American (RSNA) 2011.
September 27, 2011 – Calgary Scientific Inc. has received clearance from the U.S. Food and Drug Administration (FDA) to market medical imaging application, ResolutionMD Mobile, as a mobile diagnostic application in the United States. With this secure, highly scalable, server-based software solution, physicians anywhere can rapidly access, view and interact with patient images and reports stored within any healthcare facility, and render a clinical diagnosis using their mobile devices. Prior non-diagnostic versions of this product are currently distributed and licensed under original equipment manufacturer (OEM) agreements with global leaders in medical imaging and information technology. In addition to the recent FDA clearance, ResolutionMD Mobile has also been licensed by Health Canada and bears the CE mark for distribution in Europe.
September 27, 2011 – Fovia Medical Inc., a provider of volume-rendering technology, and Merge Healthcare, a provider of enterprise imaging and interoperability solutions, announced that Merge will enhance and standardize its product offering by incorporating Fovia’s High Definition Volume Rendering (HDVR) technology across its entire radiology picture archive and communications (PACS) platform.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Toshiba will unveil a new work in progress pediatric coil suite for its Vantage Atlas and Titan MR systems. The new coils are optimized for pediatric patients, producing better image quality for body, spine and neuro imaging.
September 27, 2011 – Continual visual verification that a tumor is being accurately targeted during a radiotherapy treatment is now possible with recent enhancements to the TrueBeam system. This capability, called intrafraction motion review (IMR), is among the innovations that Varian Medical will showcase at this year's annual meeting of the American Society for Radiation Oncology (ASTRO) in Miami Beach next week.
Toshiba will showcase its proprietary non-contrast magnetic resonance (MR) angiography imaging techniques and a new ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
Toshiba will showcase the Vantage Titan 3.0T MR system at the 2011 Radiological Society of North America (RSNA) trade show. The machine is equipped with multi-phase transmission technology, creating the most homogeneous abdominal and pelvic images on 3.0T systems.
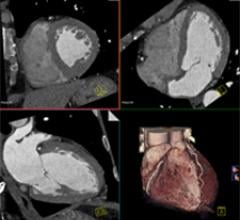
September 27, 2011 — A study in the Sept. 27 issue of the Journal of the American College of Cardiology suggests that early coronary computed tomographic angiography (CCTA) is preferable to rest-stress myocardial perfusion imaging (MPI) when evaluating acute low-risk chest pain in the emergency department.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Eizo Nanao Technologies Inc. announced it has received 510(k) clearance from the U.S Food and Drug Administration (FDA) for its RadiForce LS560W monitor with LMM 56800 medical display system.
September 26, 2011— Infinitt North America said its Xelis Fusion software has received clearance from the U.S. Food and Drug Administration (FDA), and is now widely available to the North American market.
September 26, 2011 — Hoag Breast Care Center recently became the first breast care center in California and one of a handful in the United States offering 3-D digital breast tomosynthesis for breast cancer screenings. The technology promises to improve breast cancer detection, especially in young women and women with radiographically dense breast tissue.
September 26, 2011 — Elekta will highlight several of its radiation therapy products at the American Society for Therapeutic Radiology and Oncology (ASTRO) annual meeting Oct. 2-6 in Miami Beach, Fla.
September 26, 2011 — Civco Medical Solutions is hosting a motion management symposium in conjunction with the American Society for Therapeutic Radiology and Oncology (ASTRO) 2011 conference. The symposium, "The Future of Motion Management from Micro to Macro," will be held Monday, Oct. 3, at the Gansevoort Hotel in Miami Beach.
When volumetric scanning came along a few years ago, proponents hawked the technology as a leap forward in efficiency and accuracy. Simply sweeping the entire heart would ensure the capture of every bit of data needed to make an accurate diagnosis, allowing the patient to head home, while the next hopped up on the table. It kindled expectations of an echocardiographic nirvana with unprecedented reproducibility regardless of the sonographer’s skills, skyrocketing throughput and off-the-chart revenues. Reality has fallen a tad short.

 September 28, 2011
September 28, 2011