Oct. 20, 2011 — The Radiosurgery Society (RSS), a nonprofit organization of medical professionals dedicated to advancing the science and clinical practice of stereotactic body radiotherapy (SBRT) and radiosurgery (SRS), will hold its scientific meeting Feb. 23-25 at the La Costa Resort in Carlsbad, Calif.
October 19, 2011 – Boston Scientific has completed enrollment in a clinical trial to evaluate its WallFlex Biliary RX fully covered stent for the treatment of benign bile duct strictures (narrowing or blockages). This multi-center, prospective study enrolled 187 patients at 13 centers in 11 countries worldwide. Lead investigators in the study are Professor Jacques Deviere, M.D., Ph.D., of Hospital Erasme in Brussels, and Professor Guido Costamagna, M.D., of Policlinico A. Gemelli in Rome.
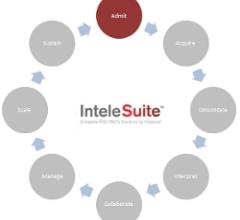
October 19, 2011 – Intelerad Medical Systems announces the launch of InteleSuite, a complete and customizable set of radiology information system/picture archiving and communications system (RIS/PACS) solutions designed to streamline workflow and augment productivity. InteleSuite creates a new alternative that combines the KLAS Ambulatory category leader IntelePACS, with Intelerad’s new InteleRIS offering.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
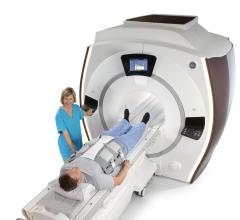
October 19, 2011 — GE Healthcare announced the United States launch of the GEM Suite (Geometry Embracing Method) of surface coils, designed for use with the Optima MR450w 1.5T wide bore magnetic resonance imaging (MRI) system. Syracuse Orthopedic Solutions is the first United States site to install the technology.
October 19, 2011 — Carestream Health announced enhancements to its image acquisition software used with Directview Vita/Vita LE/Vita SE and Point-of-Care computed radiography (CR) systems. The new software enhances workflow and offers advanced processing capabilities.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
October 19, 2011 — Fujifilm Medical Systems U.S.A. Inc. announced the commercial availability of the FDR D-EVO Wireless 17 x 17 inch flat panel detector (FPD).
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
October 19, 2011 — RamSoft’s PowerServer 5.1 has achieved 2011/2012 Complete EHR ONC-ATCB Certification, designating the software as capable of supporting providers with Stage 1 meaningful use (MU) measures. These measures are requirements to qualify for funding under the American Recovery and Reinvestment Act (ARRA).
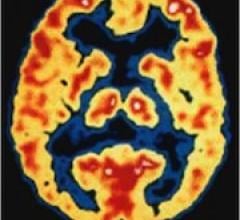
October 19, 2011 – The development of Alzheimer’s disease drugs and early detection has been hindered for years because no imaging agents or diagnostic tests existed. The only definitive way to diagnosis the disease was through cadaver brain biopsy samples. However, new radiotracers have been developed to illuminate the beta amyloid plaques that accumulate in specific areas of the brain in Alzheimer’s patients.
At RSNA 2011, Siemens will showcase its two newest groundbreaking Imaging IT solutions, syngo.via and syngo.plaza, representing the next generation of advanced visualization and agile PACS technologies that deliver cutting-edge functionalities to the clinical routine.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
October 19, 2011 — At RSNA 2011, Sectra will launch its OneScreen system, an online solution to identify patients in the risk group for osteoporosis. The service is especially convenient in combination with mammography.
At RSNA 2011, Sectra will highlight the latest development within its radiology IT suites, including features in its North American Radiology Information System (RIS) that support certification criteria to meet meaningful use objectives under the ARRA’s electronic health record (EHR) incentive program to increase customers’ profitability.
October 19, 2011 — University of California Davis (UC Davis) Health System, Petnet Solutions Inc. and Northern California PET Imaging Center (NCPIC) announced a partnership to establish a radiochemistry research and training facility on the university’s Sacramento campus. The facility will also produce radiopharmaceutical products used in positron emission tomography (PET) scans. Petnet Solutions is a wholly owned subsidiary of Siemens Medical Solutions USA Inc.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
October 19, 2011 — Hologic Inc. announced the six-year follow-up results from the MammoSite Registry Study continue to show promising results for breast cancer patients. The results were presented at the American Society of Radiation Oncology (ASTRO) meeting in Miami, Florida, Oct. 3. The company featured its next-generation MammoSite ML (multi-lumen) radiation therapy.
October 13, 2011 — Candelis Inc. announced it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Astra cloud-hosted suite of software services, which includes Astra Plus, Astra Lite and Astra Mobile.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
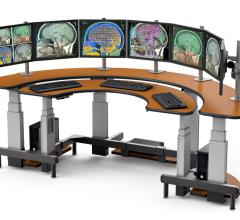
The ergonomic design of the Half-Moon-shaped Dual Tier desk from AFC Industries enables the user to arrange monitors in a way that maximizes both visibility and reach advantages. When sitting at a proper distance from the Dual Tier suited for the user, he or she gains easy access to all input entry devices. The independent electronic height adjustment capabilities of each of the surfaces allows the user to change the height of the equipment within seconds at the simple touch of a button, thus optimizing comfort in use whether in a seated or standing position.
October 18, 2011 – DR Systems will exhibit its meaningful use (MU) Imaging Electronic Health Record (EHR) a complete, cloud-based, imaging-centric ambulatory electronic health record, at RSNA 2011.
October 18, 2011 – At RSNA 2011, CoActiv debuts its Exam-RIS v.2.0, an advanced, customizable Web-based radiology information system (RIS) that brings a full range of premium features to the management of medical images, while integrating seamlessly with CoActiv Exam-PACS (picture archive and communications system). From comprehensive, specialized worklists and automated referring physician communications to a seamless HL7 interface for billing and more, new Exam-RIS, powered by Swearingen RISynergy, will help imaging sites manage workflow with complete electronic ease. The software is offered as an affordable alternative to more expensive RIS on the market.
Michael L. Steinberg, M.D., incoming president of the American Society for Radiation Oncology (ASTRO), presents ...
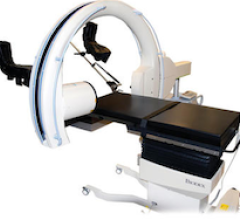
The 3-D Imaging C-Arm Table from Biodex Medical Systems is designed specifically for brachytherapy seed implantation procedures. The table features motorized actuation of height, X-Y, lateral roll and Trendelenberg motions of the tabletop.
IBA ProteusONE offers uncompromised treatment solutions enabling physicians to leverage the clinical effectiveness of ...


 October 20, 2011
October 20, 2011