Volpara breast density measurement software provides an objective, reproducible measurement of breast density designed to improve early detection among women with dense breasts. It is used by radiologists to objectively assess density and help evaluate who might benefit from additional screening.
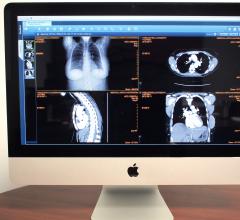
ULite is a zero-footprint viewer that enables referring physicians to access images and reports via the Web from a PC, Mac or tablet without software installation or plug-ins. ULite uses HTML5 and supports the latest versions of Internet Explorer, Safari, Google Chrome and Firefox.
The eTRAX electromagnetic needle tip tracking system is now available in 18 gauge, offering improved image-guided navigation. eTRAX allows physicians to clearly track the needle tip on screen in real-time or with preacquired 3-D volume datasets, facilitating safe and accurate instrument placement when targeting difficult-to-access lesions.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
SkyVue is a customizable cloud-based viewer platform which enables the use of one cohesive viewer within the enterprise and beyond, regardless of role or venue.
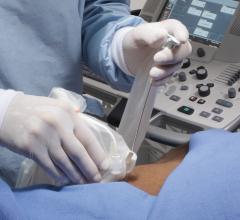
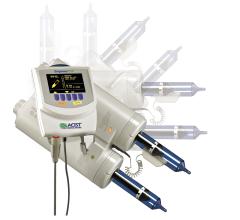
Bracco will feature the latest in contrast delivery systems at RSNA. Consistent with the theme of this year’s meeting, “Patients First,” these systems offer cutting-edge technology to impact patient care, workflow and operational efficiencies.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
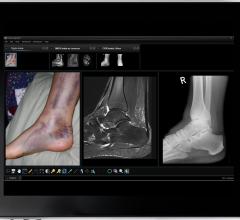
The Coronis Fusion 6 MP DL display offers multi-modality imaging to increase the productivity of radiologists. Thanks to enhanced brightness provided by new LED backlight technology, radiologists can view chest, lung, orthopedic and other types of images with clarity and precision.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Barco's Mammo Tomosynthesis 5MP display significantly improves the brightness of displays used for breast cancer detection and adds capabilities that enhance the conspicuity of the subtlest details.
November 12, 2012 — Intrinsic Medical Imaging (IMI) introduced its Matrix Volumetric Analytics software for advanced anatomical interrogation at the 24th annual Transcatheter Cardiovascular Therapeutics (TCT) conference held Oct. 23 to 26 in Miami. The technology enables assessment of vascular and heart structure pre- and post-surgery for a wide variety of procedures.
Customers of a large Australian radiology practice praise Intelerad’s referring physician portal for instant, easy access to results and images, while the practice leverages InteleConnect’s brandable and intuitive interface for new business benefit.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
GE Healthcare announced it has acquired U-Systems Inc., which recently gained U.S. Food and Drug Administration (FDA) clearance for the somo•v Automated Breast Ultrasound System (ABUS). It is the first ultrasound system on the market for breast cancer screening as an adjunct to mammography for asymptomatic women with greater than 50 percent dense breast tissue and no prior breast interventions. Financial terms were not disclosed.
iNtuition, a client-server solution for advanced image management, has been expanded with three new capabilities, including iNtuition Review. iNtuition Review enables iNtuition to support multi-monitor display and multi-modality review and is especially suited to the review of images related to conditions such as cardiac and vascular, and interventional oncology procedures.
At RSNA 2012, Philips Nuclear Medicine is introducing new clinical innovations for its positron emission tomography/computed tomography (PET/CT) systems.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
University Health System in San Antonio, Texas, was one of the first health systems in the United States to install Carestream’s new DRX-Revolution Mobile X-ray systems. The new DRX-Revolution systems are part of an order for five Carestream wireless digital radiography (DR) systems to help improve care at University Hospital in the South Texas Medical Center and a new outpatient Clinical Pavilion in downtown San Antonio.
Earlier this year the Pancreatic Cancer Action Network released a report titled, "The Alarming Rise of Pancreatic Cancer Deaths in the United States: Why We Need to Stem the Tide Today," detailing the rising threat of pancreatic cancer. The report reveals the startling observation that based on projections of U.S. population and cancer death rates, pancreatic cancer is anticipated to move from the fourth to the second leading cause of cancer death in the United States by 2020, and possibly as early as 2015.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
November 9, 2012 — The team of dosimetrists and physicists at D3 Oncology Solutions (D3), an affiliate of UPMC and UPMC CancerCenter, recently surpassed significant milestones: completing its 30,000th advanced treatment plan and commissioning its 100th linear accelerator.
Toshiba’s advanced radiation dose reduction technology, Adaptive Iterative Dose Reduction 3D (AIDR 3D), is now available ...
Toshiba will showcase Spot Fluoro, a dose management tool for Infinix-i vascular X-ray systems. Spot Fluoro allows clinicians to view a region of anatomy using live fluoroscopy while viewing the Last Image Hold surrounding area, resulting in lower dose and a larger image display area than offered by previous technology.
Offering customers CT systems to meet varying clinical needs, Toshiba showcases its Aquilion ONE, Aquilion PRIME and Aquilion RXL.
At the 2012 annual meeting of the American Society for Radiology Oncology (ASTRO) in Boston, Siemens Healthcare highlighted its technology to optimize and accurately image for radiation therapy treatment planning and follow-up.
The IMIX team will be at Radiological Society of North America (RSNA) 2012 in the South Hall, booth 3060. The latest in DR technoloy, including X Wireless, the industry's lightest wireless DR cassette, will be on display.

 November 12, 2012
November 12, 2012