Eizo Inc. recently announced that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for breast tomosynthesis for its 8 megapixel multimodality monitor, the RadiForce RX850.
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
May 4, 2016 — Infertility and hormonal fertility treatments may influence the amount of dense tissue in the breast, a ...
Today Siemens Healthcare unveiled its new brand name, Siemens Healthineers. The company said the new brand underlines Siemens Healthcare’s pioneering spirit and its engineering expertise in the healthcare industry.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
May 2, 2016 — In a new report, the U.S. Food and Drug Administration (FDA) suggests that since the passage of the ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Infinitt North America announced that the company was highlighted in the new KLAS specialty report, “Imaging IT Landscape 2016 Enterprise Platform: The Next Nirvana?”
The U.S. Food and Drug Administration (FDA) announced it is building the foundations of a national evaluation system to generate better evidence more efficiently for medical device evaluation and regulatory decision-making.
Medicine supposedly aches for a universal viewer, one that can satisfy the needs of every physician — cardiologist, radiologist, dermatologist, orthopedist; and display its images on whatever device — laptop, desktop, tablet or smartphone.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
With increased use of computed tomography (CT) scans, radiologists and other experts — including Donald Frush, M.D., medical director of the Duke Medical Radiation Center and a co-author of a new World Health Organization (WHO) report — are urging awareness and accountability around medical scans for children.
The global market for contrast media injectors is set to increase from $830 million in 2015 to almost $1.8 billion by 2022, according to research and consulting firm GlobalData.
May 3, 2016 — Good news for clinicians and their patients: the evaluation of left ventricular (LV) diastolic function ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Mirada Medical announced it will showcase its latest solutions for radiotherapy, including RTx and Workflow Box, at the European Society for Radiation Oncology (ESTRO) 2016 Congress, April 29-May 2 in Torino, Italy.
Hitachi Medical Systems America Inc. announced its All-Inclusive Service and Support Program for its Premium Performance Scenaria SE 64/128-slice computed tomography (CT) system.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Researchers with the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health, have collaborated with physicians and medical geneticists around the world to create the Atlas of Human Malformation Syndromes in Diverse Populations.
May 2, 2016 — Elekta announced that Monaco version 5.11, the latest update to its treatment planning software, is ...
In light of the 2016 Consolidated Appropriations Act, Agfa HealthCare is using its Fast Forward to DR Program to help health systems using film and computed radiography (CR) to cost-effectively upgrade their facilities to digital radiography (DR) technology.
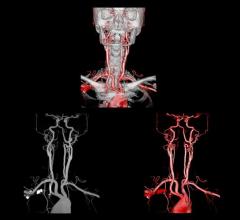
Bayer announced that the U.S. Food and Drug Administration (FDA) has approved Gadavist (gadobutrol) injection for use with magnetic resonance angiography (MRA) to evaluate known or suspected supra-aortic or renal artery disease.


 May 05, 2016
May 05, 2016