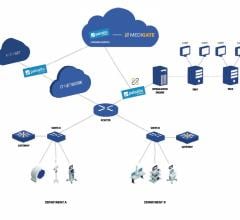
Cybersecurity company Medigate unveiled its medical device cybersecurity app for the Palo Alto Networks Application Framework. Medigate’s app will provide healthcare organizations with full visibility into the medical device inventory connected to their networks, as well as alerts to potential risks. With the capabilities of Medigate’s device security platform available via an app for the Palo Alto Networks Application Framework, customers will benefit from a comprehensive security solution that identifies and secures connected medical devices.
The American College of Radiology (ACR) Board of Chancellors has elected Geraldine McGinty, M.D., MBA, FACR, as chair. McGinty is the first woman elected chair of the board in the nearly 100-year history of the ACR.
ITN Contributing Editor Greg Freiherr interviews Kim Garriott, Principal Consultant, Logicalis The rapidly expanding ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
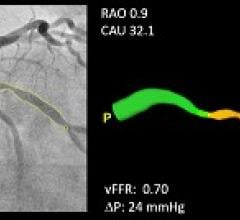
Pie Medical Imaging announced that clinical data on its CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve) software will be presented during EuroPCR 2018, May 22-25 in Paris, France. This software, which has received U.S. Food and Drug Administration (FDA) 510(k) clearance, can calculate the pressure drop and vFFR value in the coronary artery non-invasively, which means there is no need for a pressure wire and hyperemic agent.
The Istituto Oncologico Veneto (IOV) in Padua, Italy, has acquired MILabs’ latest-generation Versatile Emission Computed Tomography (VECTor5) system to support its translational oncology mission, entrusted by the Italian Ministry of Health and the Organization of the European Cancer Institutes. Due to its high-resolution imaging capabilities, MILabs’ preclinical VECTor can enable a quick translation of molecular research from the bench to the clinic at the IOV.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
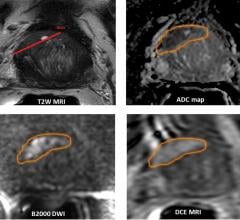
Three new studies presented at the 113th annual meeting of the American Urological Association (AUA) highlight the effectiveness of magnetic resonance imaging (MRI) in the diagnosis and management of prostate cancer. The studies concur that use of MRI in the diagnosis of prostate cancer is increasing, and brings added value to screening and surveillance.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
A new statement from the American College of Radiology (ACR) asserts that provider non-compliance to lung cancer screening guidelines may be resulting in thousands of unnecessary deaths each year. The statement attributes the phenomenon to physician ignorance of lung cancer screening guidelines, lack of patient and physician education on the benefits of screening, and drastically low Medicare reimbursement for low-dose computed tomography (LDCT) lung cancer screening exams.
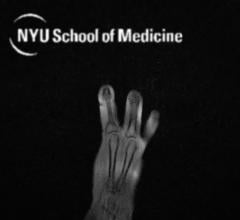
A new kind of magnetic resonance imaging (MRI) component in the shape of a glove delivers the first clear images of bones, tendons and ligaments moving together, a new study finds.
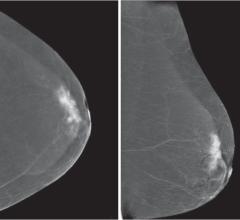
May 17, 2018 — A new JAMA Insights article notes that despite changes in mammography screening frequency recommendations ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
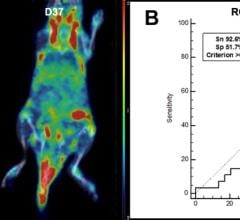
A novel positron emission tomography (PET) tracer developed by Korean researchers can visualize joint inflammation and could provide early diagnosis of rheumatoid arthritis, a common autoimmune disease that causes chronic inflammation of joints and can lead to deformity and dysfunction. The study is reported in the featured basic science article in The Journal of Nuclear Medicine’s May issue.
Enterprise imaging workflow software provider Laurel Bridge Software announced new capabilities for its Compass - Routing Workflow Manager. These include a transient and queryable cache designed to enable local study access while imaging exams reside in Compass. This will help ensure uninterrupted radiologist reading workflow when the ultimate image data source or destination is unavailable due to a LAN or WAN outage.
Denver-based Matrix Analytics announced the company's rebrand to Eon. The rebrand marks the expansion of Eon's offerings beyond LungDirect to a suite of software solutions that will help improve the treatment of complex conditions of any kind. LungDirect, which will be rebranded to EonDirect, is the first and only cloud-based software application for lung cancer screening and incidental nodule management that integrates seamlessly with hospital electronic medical records (EMRs), according to Eon.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
Accuray Inc. signed a multi-system, multi-hospital agreement with Mercy for the acquisition of Accuray Radixact and CyberKnife M6 Systems to expand access to advanced, life-saving technology for more cancer patients. The next-generation systems will replace existing Accuray devices as well as competitive conventional linear accelerators. The order was entered into backlog during the company's fourth fiscal quarter which ends June 30, 2018.
Having breast cancer placed a significantly greater financial strain on black women than white women, according to a study by researchers at the University of North Carolina Lineberger Comprehensive Cancer Center.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
May 15, 2018 — U.S. First Lady Melania Trump underwent an interventional radiology embolization procedure to treat a ...
On May 30, 2018, the American College of Radiology (ACR) Data Science Institute (DSI) and the Society for Imaging Informatics in Medicine (SIIM) will hold the Spring 2018 Data Science Summit: Economics of Artificial Intelligence (AI) in Health Care. The summit will be hosted at the SIIM 2018 Annual Meeting, May 31-June 2 in National Harbor, Md.
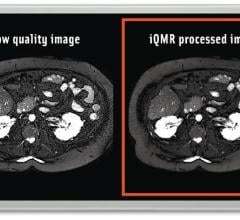
May 15, 2018 — Medic Vision Imaging Solutions Ltd. announced that the U.S. Food and Drug Administration (FDA) has ...
On April 9, 2018, the U.S. Food and Drug Administration (FDA) announced that all four of its Mammography Quality Standards Act (MQSA) accrediting bodies are approved to accredit digital breast tomosynthesis (DBT) systems. This includes the states of Arkansas, Iowa and Texas, and the American College of Radiology (ACR).
CurveBeam announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its LineUp Multi-extremity weight bearing computed tomography (CT) system.
Imaging Technology News (ITN) was honored with a pair of National Bronze Azbee Awards for editorial excellence at the 2018 American Society of Business Publication Editors (ASBPE) Azbee Awards of Excellence banquet in Washington, D.C. Both ITN awards were in recognition of the efforts of Contributing Editor Greg Freiherr — in the Regular Column, Contributed category for Freiherr’s monthly column “The Last Read,” and in the Online Video News category for his Technology Report: Enterprise Imaging from the 2016 Radiological Society of North America (RSNA) annual meeting.

 May 22, 2018
May 22, 2018