Endomag, the surgical guidance company, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to ...
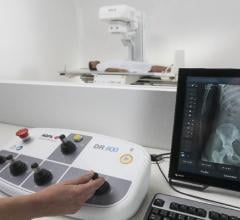
Agfa displayed the new DR 800 multi-purpose digital imaging system with Dynamic Musica at AHRA 2018. Delivering ...
Siemens Healthineers debuted the FlexForce Coach performance consulting and FlexForce Tech staffing solutions, which are ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
The use of computed tomography (CT) scans has increased dramatically over the last two decades. CT scans greatly improve diagnostic capabilities (which improve clinical outcomes) but they deliver higher radiation doses than other tests. Therefore, radiation protection is a concern, especially among children, who may receive higher radiation doses, are more susceptible to radiation-related malignancies than adults and have more time to show effects from the potential risk.
Fujifilm Medical Systems U.S.A. Inc. announced that it will offer educational opportunities and exhibit its latest innovations in digital radiography (DR), women’s health and pediatric imaging portfolio at the Association for Medical Imaging Management (AHRA) 2018 Annual Meeting and Exposition, July 22-25 in Orlando, Fla.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
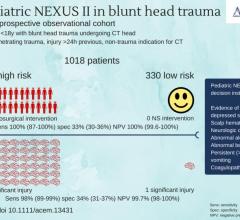
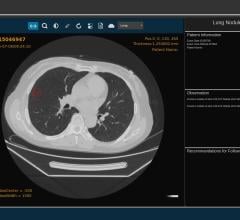
A new study finds The Pediatric NEXUS Head Computed Tomography (CT) Decision Instrument (DI) reliably identifies blunt trauma patients who require head CT imaging, and could significantly reduce the use of CT imaging. The findings of the study are published in the July issue of Academic Emergency Medicine, a journal of the Society for Academic Emergency Medicine (SAEM).
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
A novel new study provides compelling evidence that the design, development and implementation of electronic health records (EHRs) need to be improved to make them easier to use by clinicians and, ultimately, safer for patients.
A research team with funding from the National Institute for Biomedical Imaging and Bioengineering (NIBIB) has developed an advanced computing technique for rapidly and cost effectively improving the quality of biomedical imaging. The technology, called AUTOMAP, uses machine learning and software, referred to as neural networks — inspired by the brain’s ability to process information and perceive or make choices. AUTOMAP finds the best computational strategies to produce clear, accurate images for various types of medical scans.
A new way to examine stress and inflammation in the heart will help Parkinson’s researchers test new therapies and explore an unappreciated way the disease puts people at risk of falls and hospitalization.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
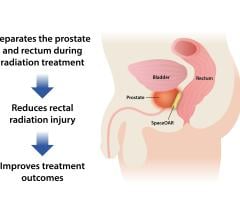
Augmenix K.K. announced that SpaceOAR hydrogel, a soft, implanted absorbable gel spacer is now available to all prostate cancer radiotherapy patients in Japan through the Ministry of Health, Labour & Welfare (MHLW) national reimbursement. SpaceOAR hydrogel is clinically proven to assist in the decrease of unwanted side effects from radiotherapy treatment in patients with prostate cancer.
A four-protein biomarker blood test improves lung cancer risk assessment over existing guidelines that rely solely upon smoking history. The blood test captures risk for people who have ever smoked, not only for heavy smokers, an international research team reports in JAMA Oncology.
At the 2018 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI), June 23-26 in Philadelphia, Siemens Healthineers announced U.S. Food and Drug Administration (FDA) clearance of syngo.via VB30 for Molecular Imaging (MI). The latest version of the company’s intelligent imaging software, syngo.via VB30 for MI has new features designed to bring advanced clinical capabilities to positron emission tomography/computed tomography (PET/CT) imaging in oncology and neurology.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
RaySearch has released RayCare 2A, the latest version of its flagship oncology information system (OIS). RayCare is designed to support the workflow in a modern oncology center, connecting the different oncology disciplines, boosting efficiency and ensuring optimal use of resources.
July 13, 2018 — Follow-up imaging for women with non-metastatic breast cancer varies widely across the country ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
iSchemaView has signed Diagnostic Imaging Australia (DIA) to be the exclusive distributor for the RAPID cerebrovascular imaging analysis platform in Australia and New Zealand. Hospitals and clinics that treat ischemic stroke in these countries will now have access to RAPID’s automated computed tomography perfusion (CTP), magnetic resonance (MR), CT angiography (CTA) and ASPECTS solutions, with support from DIA’s customer service specialists in the region.
On July 12, the Centers for Medicare and Medicaid Services (CMS) released the proposed rule for the 2019 Physician Fee ...
July 12, 2018 — Siemens Healthineers recently announced the launch of its new ultrasound system, the Acuson Sequoia. The ...
EchoNous has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the EchoNous Vein, an ultrasound-based tool designed specifically for nurses to improve peripheral IV (PIV) catheter placements. Developed for usage across a wide range of patients including both adults and children, EchoNous Vein provides immediate, clear images at depths from 1 to 5 centimeters for quickly visualizing superficial and deeper veins with just two-button controls. EchoNous Vein will integrate with the company’s existing intelligent medical tool, Uscan, to form the EchoNous platform.
Randomized clinical trials are the gold standard of cancer research and can shed light on whether innovative, new therapies with great potential actually have clear benefits over usual care for patients. However, the seven randomized trials funded by the National Cancer Institute (NCI) and the Patient Centered Outcomes Research Institute (PCORI) to test proton therapy are enrolling more slowly than expected. Commercial insurance medical policies that do not cover treatment with proton therapy can make it difficult for patients to participate in randomized clinical trials funded by the NCI, part of the National Institutes of Health, that are evaluating the therapy. That’s the message from an expert at the Perelman School of Medicine at the University of Pennsylvania and colleagues at the NCI who are calling attention to what the authors say is a major barrier these trials face. The authors publish their commentary, and proposed solutions, this week in the Journal of Clinical Oncology.
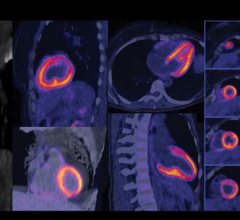
Zebra Medical Vision has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from an electrocardiogram (ECG)-gated computed tomography (CT) scan, provides physicians with important data used in the assessment of the risk for coronary artery disease.


 July 20, 2018
July 20, 2018