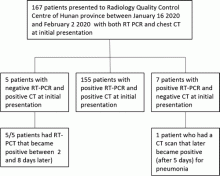
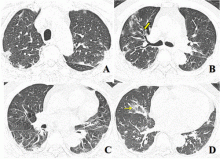
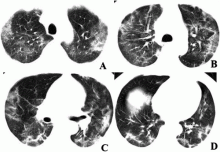
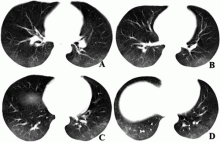
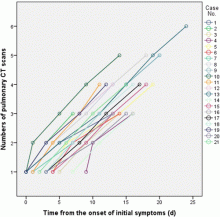
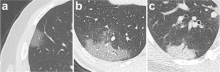
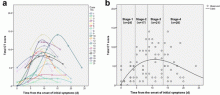
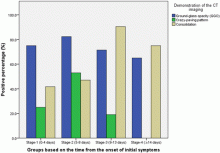
February 17, 2020 — Dicom Systems, a leader in enterprise imaging interoperability and workflow software, has announced their support of Vision Radiology in the routing, tag-morphing and management of medical imaging exams which has led to a marked improvement in diagnostic reporting and the delivery of patient care.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now