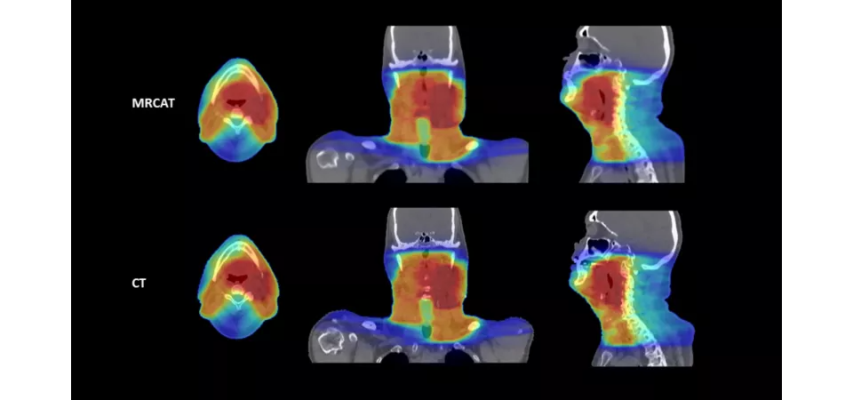
October 20, 2022 — Royal Philips, a global leader in health technology, announced two new advances in MR-only workflows to advance head and neck cancer radiotherapy imaging and simulation. The company’s artificial intelligence (AI) enabled MRCAT Head and Neck radiotherapy application, which allows the use of MR as the sole or primary imaging modality for radiotherapy planning in the treatment of soft tissue tumors in the head and neck, along with the brain, pelvis and prostate, has received FDA 510(k) clearance and is commercially available in the U.S.
As a result of a recent development partnership with patient-positioning company MacroMedics, Philips has also announced compatibility of MacroMedics’ latest DSPS (Double Shell Positioning System) Prominent positioning system [1] with Philips MR Head Neck Coil. This unique solution combines the superior soft-tissue imaging capabilities and the high-resolution image quality of Philips’ head and neck coil with the comfort-enhancing and positional accuracy and stability features of MacroMedics’ mask. These developments aim to improve the accuracy of radiotherapy planning and simulation to help achieve better patient outcomes, enhance patient comfort, and offer the efficiency benefits of an MR-only workflow.
“With this innovative mask that fits into Philips’ head coil, we expect to acquire head and neck images for radiotherapy planning with diagnostic image quality and improved patient comfort,” said Marielle Philippens, Associate Professor, MRI for Radiotherapy at University Medical Center Utrecht (Utrecht, The Netherlands), who is currently using the integrated Philips and MacroMedics solution.
“The superior soft tissue imaging of MR together with advances in the integration and orchestration of data, including the use of artificial intelligence, promise greater clarity and less subjectivity in planning radiotherapy for head and neck cancer,” said Ilya Gipp, Chief Medical Officer Oncology Solutions at Philips. “Our collaboration with MacroMedics to develop a patient-friendly mask system compatible with our high-resolution dStream imaging coils highlights Philips’ commitment to providing the precision tools needed for the localization and characterization of difficult-to-treat tumors.”
Philips’ radiation oncology portfolio is designed to provide greater clarity by empowering oncologists with accurate image guidance, integrated workflows, and the versatility to deliver the precise, adaptive, personalized treatment every patient deserves. The company’s products and services are enhanced through strategic clinical and technology partnerships, vendor-neutral device integration, advanced imaging and AI algorithms to help physicians deliver more confident diagnoses and treatment pathways for patients.
During ASTRO 2022, Philips will also highlight its strategic partnership with Elekta in precise and individualized oncology care. Building on their successful collaboration to develop Elekta Unity, the world's first high-field MR-linac (linear accelerator), the two companies are collaborating to further deliver novel image-guided adaptive, personalized treatment to help improve cancer care.
Philips is showcasing its latest innovations in radiation oncology at ASTRO 2022 (October 23-26, San Antonio, Texas, U.S.), all of which are designed to help increase accuracy across imaging, planning, and treatment; improve patient and staff satisfaction; accelerate time to treatment; and maximize investment value. Visit Philips at ASTRO 2022 for more information on Philips’ radiation oncology innovations being highlighted at the event.
For more information: www.philips.com
Find more ASTRO22 content here
[1] Pending FDA 510 (k) clearance; not for sale in the U.S.A.


 April 17, 2025
April 17, 2025 







