Incidents of cancer are on the rise globally. According to a press release from the World Health Organization’s (WHO) International Agency for Research on Cancer (IARC), “In 2012, the worldwide burden of cancer rose to an estimated 14 million new cases per year, a figure expected to rise to 22 million annually in the next decade.” As the numbers increase around the world, research continues to unveil unconventional options for cancer treatment. Over the past five years, proton therapy has emerged as one popular choice. Hailed by some as the Rolls Royce of radiation oncology, the technology’s precision and accuracy, along with its ability to prevent damage to critical structures and healthy tissues, has made it a technology that many vendors and healthcare facilities are actively investing in.
The investment in proton therapy can be seen in the recent and rapid spread of proton therapy centers in the United States and around the world. While there are already a handful of proton therapy centers in the United States, market research from Research and Markets predicted that by 2018 the United States would be home to 29 proton therapy centers, with revenue reaching $1.9 billion. Another report by ReportLinker.com, “Global Proton Therapy Outlook 2018,” revealed that 16 proton therapy centers have been established in Italy, France, Japan, Germany, China and the United Kingdom within the past five years alone.
Expanding Possible Use
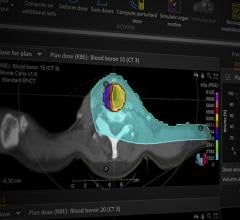
As proton therapy centers spring up around the world, clinical trials and studies are expanding the potential uses of the technology. Initial results of research being conducted at Loma Linda University Medical Center (LLUMC) in California have shown that proton therapy can successfully treat metastases to the liver by delivering higher doses of radiation than previously used with conventional X-ray therapy. “Typically invasive open surgery and chemotherapy have been the two main options for controlling liver metastases; but due to patients’ immune systems being compromised or because they are weak, aged and/or frail, many cannot withstand open surgery,” said Gary Yang, M.D., professor of radiation medicine and head of the gastrointestinal service in the Department of Radiation Medicine at LLUMC, and principal investigator of the study, “Stereotactic Body Proton Therapy for Liver Metastases.” He added, “This is what makes our study and the potential benefits of proton therapy so important. We are in Phase 1 of the study, and our initial results are looking promising. Thus far our patients are showing significant tumor reduction without adverse side effects.”
Proton therapy has already shown many advantages over other forms of radiation therapy for the treatment of complex head and neck and prostate cancers. However, recent developments are further supporting these claims. A study by radiation oncologists at Mayo Clinic showed that proton therapy significantly improved disease-free survival and tumor control when compared to intensity-modulated radiation therapy (IMRT) for advanced head and neck cancers. The researchers used a database to select nasal cavity and paranasal sinus tumor studies, and compared five-year outcomes between those patients receiving proton therapy and those receiving IMRT. The research showed that patients receiving proton therapy had a higher disease-free survival rate than those who received IMRT — 72 percent and 50 percent respectively. The researchers also found that tumor control was greater for patients receiving proton therapy not at five years, but at the longest follow-up — 81 percent versus 64 percent.
Increasing Affordability, Decreasing Size
Although proton therapy is gaining popularity, the number of proton centers currently available to treat the multitude of cancer patients in the United States and around the world is still relatively small. The equipment is an added expense, and with facilities already facing declining reimbursements, many do not have the available funds or space to create their own proton therapy centers. However, major proton therapy vendors, including Hitachi, IBA (Ion Beam Applications SA), Mevion Medical Systems and Varian, are taking note and developing more compact, efficient and affordable systems, so that more healthcare organizations can take advantage of the technology.
This year, ProTom International Inc. received U.S. Food and Drug Administration (FDA) clearance for its Radiance 330 proton therapy system, a compact, modular and more affordable proton beam delivery system. According to the company, “The system delivers extremely focused, conformal, scanned proton beams that destroy tumors while sparing nearby healthy tissue and anatomical structures.” The modular design accommodates 3-D imaging solutions and treatment planning software, and the accelerator is compact and requires 40 percent less radiation shielding than many other systems today.
IBA also received FDA clearance for its compact gantry beam line this year. The company anticipates this approval will increase international interest in its ProteusONE proton therapy compact system. It is a single-room proton therapy system that is also smaller and less expensive. In a press release, the company stated the system is “faster to install, and encompasses the latest in targeted proton therapy technologies, including intensity-modulated proton therapy.”
Support From ASTRO
Healthcare facilities interested in proton therapy are also getting support from the American Society for Radiation Oncology (ASTRO) as the society advocates for more widespread availability of the technology. A new model policy by ASTRO outlines which cancer diagnoses meet its evidence-based standards and should be covered by private insurers and Medicare. “Proton beam therapy (PBT) is demonstrating promise in our continuing efforts to improve survival and cure rates for cancer patients while reducing side effects,” said Colleen A.F. Lawton, M.D., FASTRO, chair of ASTRO’s Board of Directors. “As the leading experts in radiation oncology, it is important for ASTRO to provide balanced, evidence-based guidance to payers that ensures access to PBT for cancer patients while being judicious stewards of our nation’s and our patients’ financial resources.”

 May 06, 2024
May 06, 2024