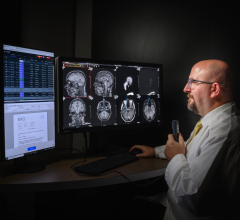
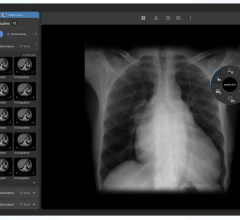
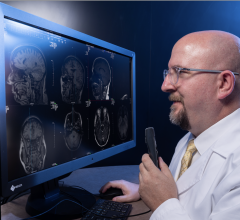
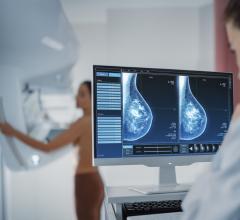
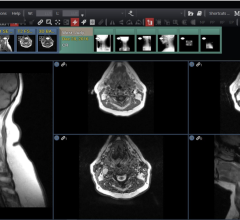
April 3, 2008 – The FDA granted 510K clearance to Kanteron Systems for marketing its KDS (Kanteron DICOM Station), offering an open source mini-PACS and multimodal workstation in the market for processing, rendering and storing digital medical images.
KDS allows users to store DICOM studies, complete drag-and-drop multimodal fusion, marking of ROIs (with templates), interconnect with other DICOM nodes for working both in a network and as a distributed computer cluster, anonymize patient's personal data (HIPPA compliant), engage in telemedicine with its integrated real-time videoconference and collaboration, and render in 3D, 4D and 5D (and in high resolution) digital medical images.
Available in the market worldwide, KDS have been installed in Australia, China, Italy, Portugal and Spain.
For more information: www.kanteron.com


 July 25, 2024
July 25, 2024