January 22, 2008 - ACR Image Metrix, a for-profit contract research organization headquartered in the American College of Radiology Philadelphia office, will conduct a multireader clinical study to determine whether the addition of the iCAD Inc. CT Colon Computer-Aided Detection (CAD) system enables radiologists interpreting CT colonography images to detect more cancers than they would without utilizing the iCAD system in the image interpretation process.
ACR Image Metrix will work with iCAD to develop and execute a clinical study to support FDA approval of CT Colon CAD.
“CT colonography is a cutting-edge technology that has the potential to save countless thousands of lives when effectively utilized. It is important to know whether adjunct technologies such as CAD can help make CT colonography even more effective at detecting colorectal cancer, the nation’s second leading cancer killer,” said Bruce J. Hillman, M.D., FACR, ACR Image Metrix director of Scientific Affairs.
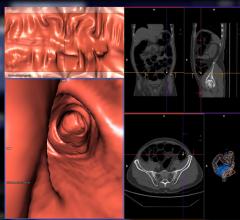
CT Colonography employs virtual reality technology to produce three-dimensional, “fly through” images that permit a thorough and minimally invasive evaluation of the entire colorectal structure for cancer and precancerous polyps. CAD computer software would then search the CT images obtained for abnormal areas of density or mass that may indicate the presence of cancer and precancerous polyps. The CAD system highlights these areas on the images, alerting the radiologist to the possible need for further analysis.
“This partnership represents a major step forward in the development of our Colon CAD product,” said Ken Ferry, president and chief executive officer of iCAD. “We feel that working with ACR Image Metrix, a group with significant expertise in radiology clinical trial management, will put us in an optimal position to submit solid clinical data to FDA and meet our goals for this important product line.”
The recently presented results of the American College of Radiology Imaging Network’s National CT Colonography Trial, along with a number of published studies, indicate that CT colonography is a viable colorectal cancer screening alternative and less invasive than the “gold standard“ optical colonoscopy. Legislation was recently introduced to the U.S. House of Representative that would provide mandatory Medicare reimbursement to providers of CT Colonography.
“It is clear from the low compliance levels that we need more options in colon cancer screening,” said Dr. Hillman. “We feel that CT Colonography has the potential to drastically improve colon cancer screening and we look forward to exploring how CAD can impact and enhance this new option for detecting cancer.”
For more information: www.imagemetrix.acr.org, www.icadmed.com

 February 06, 2024
February 06, 2024 








