January 15, 2016 — Volpara Solutions announced that it has received a new 510(k) clearance from the U.S. Food & Drug Administration (FDA) for Volpara Density Maps. The new solution is designed to help radiologists address the requirement in the Breast Imaging Reporting and Data System (BI-RADS) 5th Edition Atlas to provide “an overall assessment of the volume of attenuating tissues in the breast, to help indicate the relative possibility that a lesion could be obscured.”
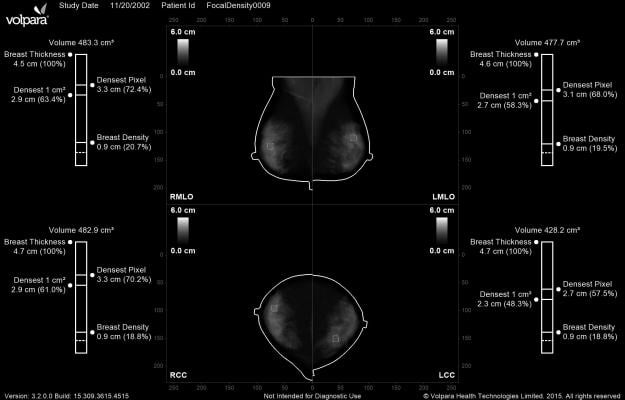
Volpara Density Maps present the 3-D quantitative volumetric information created by the VolparaDensity algorithm in a 2-D image that is available as an additional DICOM secondary capture image. Going beyond the measurement of the area of dense fibroglandular tissue, VolparaDensity measures the compressed thickness and volume of dense tissue, which enables the software to highlight specific parts of the breast tissue that are especially dense, including marking the densest 1-2 cm region of the image that might warrant closer inspection due to the masking risk.
VolparaDensity results have been clinically validated in independent studies to correlate to mammography sensitivity. Results of the study “Quantifying the Potential Masking Risk of Breast Density in Mammographic Screening,” conducted by researchers from Elizabeth Wende Breast Care (EWBC), demonstrated that VolparaDensity captures the potential masking risk of breast density more precisely compared to the widely used BI-RADS density categories. Additionally, several studies using Volpara Density Maps presented at the Radiological Society of North America (RSNA) 2015 annual meeting indicate promise for identifying those women at risk of a masked cancer. In the study, “How Can We Identify Women at Risk for a Masked Cancer, Who May Benefit from Supplemental Screening?,” researchers from Nijmegen used novel masking measures derived from density maps to help identify women at risk of a masked cancer.
Beyond masking risk, “For Presentation” images used for cancer detection are often displayed zoomed in, with heavy image processing, making it difficult to judge breast density, especially density changes over time. Volpara Density Maps are designed to show true breast density at true size, providing a standardized means of visually assessing which regions of dense tissue change over time.
Volpara recently announced another 510(k) clearance from the FDA for VolparaDensity covering VolparaDensity version 3.1, which has been specifically designed to correlate to the Fifth Edition of the BI-RADS Atlas from the American College of Radiology (ACR). VolparaDensity v3.1 is the first density assessment tool available clinically for use with digital breast tomosynthesis data sets from Hologic, GE and Siemens tomosynthesis systems, according to Volpara.
For more information: www.volparasolutions.com


 March 21, 2025
March 21, 2025 








