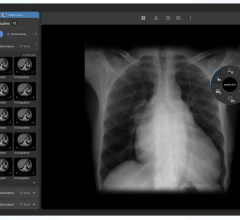
January 7, 2015 — Viztek announced upgraded features that have been added to its Exa PACS. The new PACS (picture archive and communication system) platform was designed to be fully Web-based on any device with any operating system, and increase the speed and tool set from which radiologists and referring physicians are able to access radiology exams. Exa PACS was demonstrated during the Radiological Society of North America’s (RSNA’s) 100th annual Scientific Assembly and Annual Meeting in Chicago.
Key features and benefits of the Exa PACS include the following:
- Zero footprint viewing – Regardless of location, radiologists and referring physicians will benefit from the pending zero footprint, diagnostic- quality viewing for any modality. There are no plug-ins required, no limitations, and full functionality is available regardless of operating system or age of the computer. Additionally, a fully integrated dictation module is seamlessly enabled.
- Server-side rendering – Image download time is enhanced, particularly for mammography, positron emission tomography/computed tomography (PET CT), 3-D and other large image files, especially in multi-site or distributed environments. Server-side rendering also helps to extend the life of older workstations with less processing capability.
- Automatic anatomical linking of all priors – Radiologists can save precious time and be assured of accuracy with the ability to automatically cross-link and synchronize CT priors. Guesswork and human error is eliminated. For any CT study, such as when viewing critical pathology like the size of cancerous nodules, the software automatically anatomically indexes all priors – regardless if there is one or are five or more. Fast, accurate viewing can be relied upon.
The Viztek Exa PACS works in tandem with the Exa electronic health record (EHR), which has bundled all essential components of the radiology workflow process including PACS, practice management, billing, order entry, radiology reports, patient portals and other software product currently delivered by the company.
Exa PACS is pending U.S. Food and Drug Administration (FDA) 510(k) clearance and is not yet available for sale.
For more information: www.viztek.net


 November 29, 2025
November 29, 2025