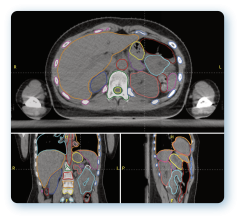
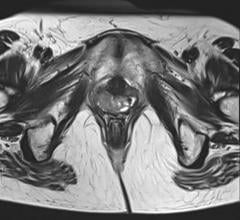
November 6, 2012 — Varian Medical Systems showcased the latest version of the Vitesse real-time treatment planning for high-dose-rate (HDR) brachytherapy at the annual meeting of the American Society for Radiation Oncology (ASTRO). This software will make it possible for clinicians to plan and perform high-dose-rate (HDR) brachytherapy treatments accurately and quickly in the operating room using real-time ultrasound guidance, without moving the patient to a computed tomography (CT) scanner partway through the process.
"With this new version of Vitesse, we have reduced the number and complexity of steps involved in planning an HDR brachytherapy treatment," said Hosea Mitchell, general manager of Varian BrachyTherapy. "We have eliminated the need to export data to another software program to finalize the plan, making the process cleaner, and easier to learn and execute. Clinicians will be able to complete the whole process within one integrated workflow, from capturing an ultrasound image to finalizing an approved treatment plan."
HDR brachytherapy involves the delivery of radiation therapy through the temporary placement of tiny radioactive sources directly into the targeted area. Using a robotic device called an afterloader, clinicians place a wire that has a radioactive source at its tip into position through needles that have been inserted into the area to be treated. Under computer control, the source is then moved within the needles to create the specified dose distribution within the patient's anatomy. The wire is then retracted into the afterloader.
Using the latest version of Vitesse, clinicians will more easily complete two brachytherapy treatment fractions in one day, rather than having the patient stay in the hospital for treatments spread over multiple days. Treatment plans, which specify the precise placement of sources within the needles as well as the duration of dwell time in each location, can be created in a real-time environment, using ultrasound images generated in the operating room rather than requiring that the patient be moved to a CT scanner room for X-ray images.
"The use of the newest version of Vitesse will enable clinicians to reduce the amount of time patients must spend waiting for treatment once needles have been implanted by as much as an hour and a half," said Tim Clark, marketing manager for Varian BrachyTherapy. "And by not having a CT scanning step, we reduce the number of times a patient must be moved, simplifying the logistics of an HDR brachytherapy treatment."
Vitesse 3.0 is currently pending FDA 510(k) clearance and not available for sale in the United States.
For more information: www.varian.com


 July 11, 2024
July 11, 2024