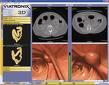
December 8, 2008 - Viatronix Inc., a developer of 3D imaging software, reported it received FDA 510(k) market clearance for its V3D-Cardiac module.
Viatronix developed the module utilizing the Viatronix V3D user-friendly interface and workflow. The V3D-Cardiac application combines left ventricle and coronary analysis functions into a single “user friendly” module. Since both functions are analyzed in a single application, the physician can now choose from any of the multiple phases when performing a coronary analysis.
All of Viatronix’s V3D clinical applications are available on an enterprise wide “software only” server-based licensing model. In the near future we expect to deliver other organ specific modules to meet the demands of the diagnostic imaging market.
For more information: www.viatronix.com

 February 01, 2024
February 01, 2024