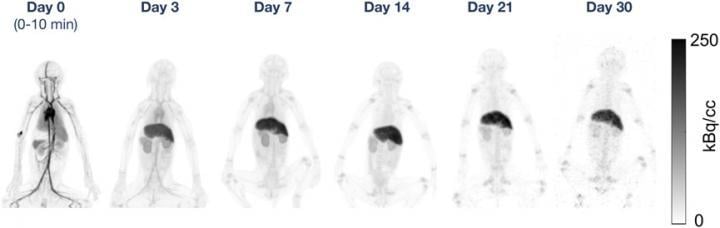
Maximum-intensity PET projections at each time point for one rhesus monkey in the 89Zr-DFO-squaramide-anti-gD group. Image courtesy of Eric Berg, University of California, Davis, CA
April 10, 2020 — Combining 89Zr-labeled antibodies with total-body positron emission tomography (PET) has extended the utility of novel total-body PET scanners, providing suitable images up to 30 days after the initial injection. A new study, published in the March issue of the Journal of Nuclear Medicine, compared four different types of 89Zr-labeled antibodies in preclinical trials, noting excellent consistency for each radiotracer even at very late time points, as well as differences in antibody behavior that are critical to understanding future outcomes of total-body PET in humans.
Monoclonal antibodies — laboratory-developed proteins designed to recognize specific targets — have been used in medicine for decades to treat various diseases, such as cancer, rheumatoid arthritis, multiple sclerosis and cardiovascular disease. Many new engineered monoclonal antibodies have been developed with specific molecular functions in order to achieve a balance between potency and safety in patient treatment.
"The tissue biodistribution of these newly engineered antibodies must be measured in vivo over the course of weeks to select the most appropriate candidates for novel therapeutics and understand how to use them in humans to best effect," said Simon Williams, Ph.D., principal scientist, molecular imaging at Genentech, Inc. "In our study, we assessed the feasibility of using a novel total-body PET scanner to image 89Zr-labeled antibodies up to 30 days after injection, allowing for the measurement of tissue biodistribution over a long period of time."
Researchers prepared four types of 89Zr-labeled antibodies, each with a different chelator-linker, to be compared across 12 young rhesus monkeys (three animals for each chelator-linker combination). Each group of animals received an intravenous injection of one of the four 89Zr-labeled antibodies in the left arm, along with an additional mass dose of unlabeled antibodies in the right arm. The animals were imaged on the day of injection, as well as three, seven, 14, 21 and 30 days after the injection.
Excellent image quality was obtained on the initial total-body PET scans for all four types of 89Zr-labeled antibodies. Results from the 30-day scans showed image quality across the four antibody types sufficient to readily identify activity in the liver, kidneys and upper and lower limb joints. However, significant differences in uptake between the various chelator-linker combinations were noted in the late time point liver, bone, and in whole-body clearance. These differences were determined to be partly related to the stability of the radiolabeled compounds prior to injection.
"The results of this study have two key implications for the field of molecular imaging," said Simon R. Cherry, Ph.D., distinguished professor at the University of California, Davis. "First, and most obvious, is that the increase in sensitivity of total-body PET, when compared to conventional PET, enables radiotracers to be followed for a longer period of time thus extending the imaging window. Using 89Zr as the radiolabel, this allows the assessment of slow biological processes and the ability to determine the ultimate fate of agents introduced into the body over a one-month timeframe."
He continued, "Second, studies with 89Zr can be conducted with much lower injected doses of radioactivity. The late time point total-body imaging conducted in this study clearly demonstrates that acceptable quality imaging with 89Zr can be accomplished when there is as little as 1/100th of the activity remaining in the subject. This paves the way for broad and repeat use of 89Zr-radiolabeled tracers in patients with extremely low effective doses."
The authors of "Total-Body PET and Highly Stable Chelators Together Enable Meaningful 89Zr-Antibody PET Studies up to 30 Days After Injection" include Eric Berg, Department of Biomedical Engineering, University of California-Davis, Davis, California; Herman Gill, Jan Marik, Annie Ogasawara and Simon Williams, Department of Biomedical Imaging, Genentech Inc., South San Francisco, California; Guus van Dongen and Daniëlle Vugts, Department of Radiology and Nuclear Medicine, Amsterdam UMC, VU University, Amsterdam, The Netherlands; Simon R. Cherry, Department of Biomedical Engineering, University of California-Davis, Davis, California, and Department of Radiology, School of Medicine, University of California-Davis, Davis, California; and Alice F. Tarantal, Department of Pediatrics and Department of Cell Biology and Human Anatomy, School of Medicine, and California National Primate Research Center, University of California-Davis, Davis, California.
For more information: www.snmmi.org


 July 30, 2024
July 30, 2024 








