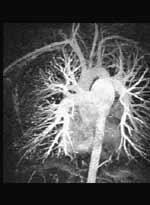
July 12, 2011 – To streamline diagnosis and enable greater overall exam efficiency, Toshiba America Medical Systems Inc. has announced U.S. Food and Drug Administration (FDA) approval of VirtualExplorer Version 3.1, a software upgrade package for Toshiba Magnetic Resonance (MR) VirtualExplorer workstations. The software provides enhanced post-processing functionality, and the new cardiac package includes MR flow analysis, which allows clinicians to measure flow and velocity when imaging the heart.
The software includes features such as 3-D image visualization, DICOM viewer, 2-D filming, double oblique and virtual endoscope. The cardiac edition of the software upgrade also includes features specific to cardiac imaging, such as MR flow analysis, MR cardiac analysis, MR coronary analysis and delayed enhancement. Additionally, the neuro edition of the software includes brain perfusion for neuro evaluation.
For more information: www.medical.toshiba.com


 July 25, 2024
July 25, 2024 








