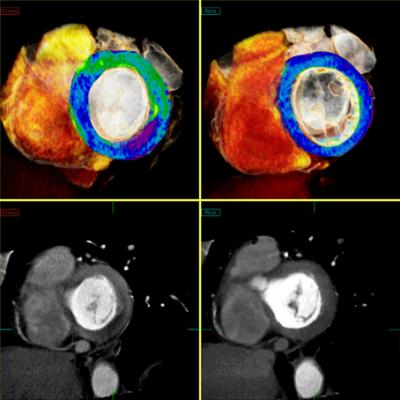
March 12, 2014 — The U.S. Food and Drug Administration (FDA) cleared Toshiba’s CT Myocardial Perfusion capability. Available on Toshiba’s Aquilion One and Aquilion OneVison Edition CT systems, Myocardial Perfusion allows clinicians to visualize myocardial ischemia with CT, providing a clinical and operational solution to make work flow.
The Myocardial Perfusion on Toshiba’s 320-detector-row CTs shows blood flow and anatomy within the coronary arteries to help determine the viability of the heart muscle. This enables clinicians to make faster and more accurate decisions on whether to undergo revascularization of coronary blockages. Toshiba’s CT Myocardial Perfusion puts patient experience first with shorter exam times and significantly lower radiation exposure.
Toshiba will showcase its Myocardial Perfusion technology at the American College of Cardiology (ACC) annual meeting in Washington, D.C., March 29–April 2 (Booth #2323).
For more information: www.medical.toshiba.com


 August 09, 2024
August 09, 2024