August 28, 2008 – TomoTherapy reported that the FDA has granted 510(k) clearance for its radiation therapy technology, TomoDirect, designed to complement helical tomotherapy and faster treatment planning.
Tomotherapy will unveil TomoDirect at the European Society for Therapeutic radiology and Oncology (ESTRO) in Gothenburg, Sweden, Sept. 14-18, 2008, and the American Society for Therapeutic radiology and Oncology (ASTRO) in Boston, MA, Sept. 21-25, 2008.
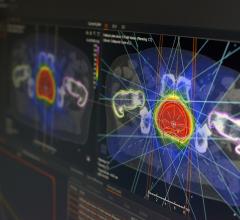
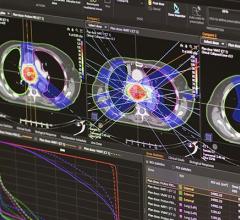
The solution is a new discrete-angle, sliding-beam delivery mode for the Hi·Art treatment system. With this capability, clinicians gain an efficient complement to helical TomoTherapySM, one that enables fast treatment planning and delivery, while broadening the spectrum of patients that can be optimally treated with the industry’s most advanced platform for cancer care.
“This is a major milestone for TomoTherapy and, in turn, for cancer centers of all sizes,” said Fred Robertson, CEO of TomoTherapy. “TomoDirect significantly improves the throughput of our system, and widens the TomoTherapy treatment options available for clinical use. Now, more cancer patients can benefit from true, next-generation treatment technology.”
TomoDirect was developed as a complement to helical TomoTherapy, with both modes utilizing the same binary multi-leaf collimator and CT-style gantry technology, and sharing a simple, consistent treatment planning and delivery process. The choice of which modality to use for a given case will depend on the nature of the tumor volume and surrounding organs at risk. TomoDirect allows clinicians to choose several discrete angles as well as the optimal modulation level required for delivery. It is expected to provide significant time savings in both the planning and delivery phases for several clinical scenarios, including whole breast irradiation and palliative treatments.
In addition to the added capabilities offered by TomoDirect, the Hi·Art system’s treatment modes are being expanded to include 3D conformal delivery, thereby providing a comprehensive range of options for all clinical cases.
For more information: www.tomotherapy.com

 June 19, 2024
June 19, 2024