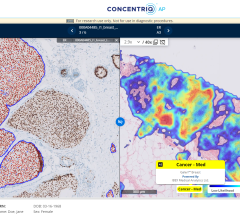
January 12, 2021 — Therapixel announced that MammoScreen has successfully obtained the CE mark. After having received FDA clearance for commercial sales in the United States in March 2020, this artificial intelligence (AI) solution is now available in the European market.
MammoScreen automatically analyzes screening mammograms and indicates suspicious mammograms and lesions to the radiologist. According to the probability of malignancy and the degree of confidence of the algorithm, the software assigns a unique "MammoScreen Score" ranging from 1 to 10.
A clinical study conducted in 2019 and published in Radiology: AI, showed that radiologists assisted by MammoScreen have a better performance than without the software.
MammoScreen helps to increase the interpretation speed of benign images, giving readers more time to focus on cases requiring more attention.
Already installed in 14 hospitals and radiology practices in France for clinical evaluations, MammoScreen has generated a lot of enthusiasm among radiologists. Physicians perceive it as an intuitive and easy-to-use tool that helps reassure themselves in the interpretation and avoid missing cancers.
Breast cancer is one of the most common and deadliest cancers in women worldwide, affecting 1 in 8 women in their lifetime. The earlier this disease is diagnosed, the more effective the treatment is.
"We are proud to have received the CE Mark. This brings us a step closer to our goal of reducing breast cancer burden worldwide by improving breast cancer screening," said Pierre Fillard, Therapixel's Founder and Chief Scientific Officer.
"I am confident that MammoScreen will not only increase radiologists' performance but will also improve the patient experience by reassuring women faster," stated Matthieu Leclerc-Chalvet, CEO of Therapixel.
For more information: www.mammoscreen.com

 August 29, 2024
August 29, 2024