September 6, 2007 – According to a new report by Datamonitor, significant progress is being made with research into the disease and innovative new therapies may be able to improve survival of brain cancer patients.
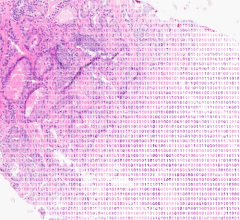
The most aggressive and most common form of brain cancer is known as glioblastoma multiforme (GBM), accounting for over half of all brain tumors diagnosed each year. Datamonitor predicts that there will be around 26,000 new cases of GBM diagnosed in the seven major markets in 2007. In the US alone, around 14,000 people will die from brain cancer in 2007.
For GBM patients, standard treatment currently involves surgical removal of the tumor followed by radiotherapy and chemotherapy. The favored brand of chemotherapy for most physicians is Schering-Plough’s Temodar (temozolomide). While survival is low for most GBM patients treated in this way (on average, just over 14 months), Datamonitor found in its research that this mode of treatment was commonly used in all of the seven major markets.
In comparison to some other types of cancer, brain tumors are less prevalent. Therefore, from a drug developer’s point of view, targeting the disease may not be considered as commercially viable compared to the four major types of cancer - breast, prostate, lung and colorectal cancer.
However, there are several incentives for drug developers to focus on brain cancer. The poor survival prospects with currently available treatment options mean that there could be rapid uptake of a new brain cancer drug providing just a modest improvement in survival. There is also a significant opportunity in the market for a second-line therapy to be used if and when the disease relapses after standard treatment fails, according to Datamonitor oncology analyst Dr. Tom Gray.
Consequently, there has been a considerable amount of research and development activity in brain cancer, and there are several innovative drugs currently in the development pipeline. Many of these drugs are targeted therapies which, rather than indiscriminately destroying cells like traditional chemotherapy, are intended to specifically destroy or prevent the growth of cancer cells.
Some of the more promising targeted therapies in the pipeline for brain cancer include Genentech/Roche’s Avastin (bevacizumab) and AstraZeneca’s Recentin (cediranib) which are currently in Phase II clinical trials. These drugs both work by preventing the growth of new blood vessels from the tumor, therefore starving it of oxygen and nutrients and preventing it from growing, Dr. Gray says.
However, as these drugs have not yet entered Phase III trials, it is likely to be at least another two years before these drugs are available to brain cancer patients.
While there is generally a great deal of optimism amongst brain cancer experts surrounding targeted therapies, it appears unlikely that Temodar will be superseded in the near future as the number one drug therapy for brain cancer. Physicians interviewed by Datamonitor envisage targeted therapies being used alongside Temodar, rather than directly replacing it.
Advances in diagnostic methods mean that a more detailed picture of the nature of brain cancer is beginning to emerge. For example, it is now known that certain proteins are present in the tumor cells of some brain cancer patients, but not in others, due to the differing genetic characteristics of these patients. Consequently, it is likely that some patients will benefit more than others from particular treatments.
For more information: www.datamonitor.com


 November 11, 2025
November 11, 2025