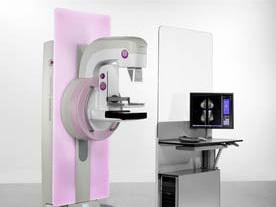
July 2, 2014 — Siemens Healthcare recently submitted its premarket approval application (PMA) for the Mammomat Inspiration with Breast Tomosynthesis option to the U.S. Food and Drug Administration (FDA).
In this PMA submission, Siemens Healthcare has provided the FDA with clinical study results and manufacturing information. The Siemens Breast Tomosynthesis option has been designed as an add-on option for the Mammomat Inspiration digital mammography system. In tomosynthesis mode, the Mammomat Inspiration acquires 25 projection views over an angular range of 50 degrees to produce 3-D digital breast tomosynthesis (DBT) images. These images are intended to be suitable for the screening and diagnosis of breast cancer.
The Siemens Breast Tomosynthesis option has been commercially available and used clinically for diagnosis since 2009 in Europe, Asia and South America. In the United States, the FDA classifies breast tomosynthesis as Class III, meaning that data from a clinical trial is required to measure safety and effectiveness. Class III devices require a PMA submission to the FDA for approval.
For more information: www.siemens.com/healthcare


 July 29, 2024
July 29, 2024