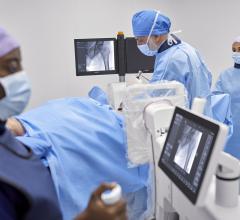
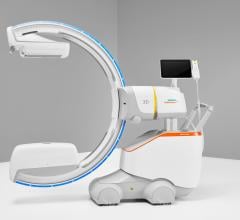
March 14, 2016 — Siemens Healthcare announced the U.S. Food and Drug Administration (FDA) has cleared three brand-new system models in the company’s Cios family of mobile C-arms – Cios Fusion, Cios Connect and Cios Select. These new systems join a newly upgraded version of Siemens’ established, ultra-premium Cios Alpha mobile C-arm.
Offering the state-of-the-art flat panel detectors of the Cios Alpha in the medium price segment, the new Cios Fusion mobile C-arm has two detector sizes – 30 cm x 30 cm and 20 cm x 20 cm. It offers most of the same software and hardware features of the Cios Alpha, including a touchscreen remote that can be positioned at the operating table to control the C-arm from within the sterile work area.
The new Cios Connect mobile C-arm weighs just 547 lbs. and enables surgeons to perform a broad area of applications. Software options allow physicians to display individual images simultaneously in subtracted and X-ray views, to optimize the presentation of contrast and bone, and support a broad range of surgical needs.
The new entry-level, user-friendly Cios Select mobile C-arm boasts an easy-to-use push button interface for fast workflow. It uses the image processing algorithm IDEAL (Intelligent Dose Efficiency Algorithm) to provide continuous contrast and brightness adjustments as well as the automatic dose-performance adjustment that is available across the entire Cios family.
The new version of Siemens’ established Cios Alpha mobile C-arm features a square flat-panel detector that helps users reach optimum levels of minimally invasive intervention in the modern surgical environment. Revised software delivers a large preview image that allows operating room staff to select appropriate image settings more easily. A new one-touch metal correction function compensates for metal image components to enable representation of surrounding tissue with greater contrast. And to mark anatomical structures, the Cios Alpha’s Live Graphical Overlay function, which formerly had been available exclusively to vascular surgery users, now can be used in all operating modes.
For more information: www.usa.healthcare.siemens.com


 August 14, 2025
August 14, 2025