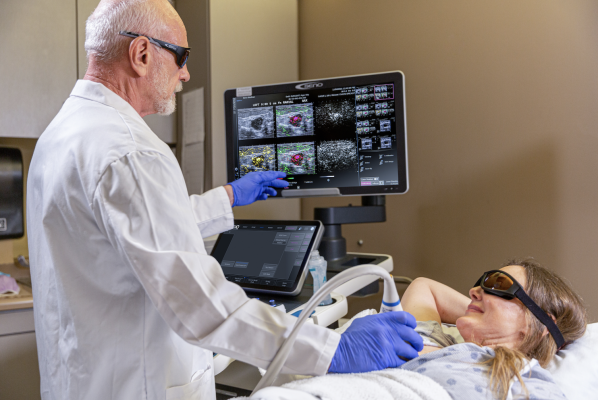
The Imagio Breast Imaging System helps physicians differentiate between benign and malignant breast lesions using non-invasive opto-acoustic/ultrasound (OA/US) technology to provide information on breast lesions in real time, helping providers to characterize and differentiate masses that may — or may not — require more invasive diagnostic evaluation.
July 26, 2022 — Seno Medical’s Imagio Breast Imaging System, a revolutionary new modality in breast imaging, has received supplemental premarket approval (PMA) from the Center for Devices and Radiological Health (CDRH) of the US Food & Drug Administration (FDA). Seno’s market-ready, groundbreaking diagnostic breast cancer imaging system helps physicians differentiate between benign and malignant breast lesions using non-invasive opto-acoustic/ultrasound (OA/US) technology to provide information on breast lesions in real time, helping providers to characterize and differentiate masses that may — or may not — require more invasive diagnostic evaluation.
The Imagio Breast Imaging System incorporates state-of-the-art ultrasound imaging technology required for premier breast imaging centers, as well as advanced ultrasound technology integrated into the opto-acoustic probe, a new ultrasound probe, and elimination of redundant electronics, making this version of Imagio more ergonomic and truly disruptive for the marketplace with the latest technological advances.
Tom Umbel, President and CEO of Seno Medical, commented, “This team is passionately committed to providing a revolutionary leap forward in patient care, and our commercial version of our Imagio System delivers just that. With our novel OA/US platform, combined with the latest technologies available today in breast imaging, we are delivering a new hybrid modality that will help providers deliver the best care possible to their patients.”
The company is launching a mobile education and demonstration tour, Imagio OA/US Road Show - Scans Across America, to enable on-site, hands-on demonstration with the Imagio System throughout the United States. The Imagio OA/US Road Show - Scans Across America tour will begin in late July with continued stops across the countbreastry throughout 2022.
Breast biopsy procedures, caused by false-positive diagnostic assessments, cost the US healthcare system more than $2 billion per year.[i] Seno’s Imagio technology could significantly reduce those costs with its non-invasive OA/US innovation.
Seno’s OA/US system combines laser optics and grayscale ultrasound to provide fused functional and anatomical breast imaging. The opto-acoustic images provide a unique blood map in and around breast masses, while the ultrasound provides a traditional anatomical image. Through the appearance or absence of two hallmark indicators of cancer — angiogenesis and hypoxia — Seno Medical has shown that the Imagio OA/US Breast Imaging System will be a more effective tool to help radiologists confirm or rule out malignancy compared with traditional diagnostic imaging modalities. And it does this without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. In addition to the novel imaging provided by the Imagio System, Seno includes an artificial intelligence (AI) decision-support tool (SenoGram) to aid physicians in interpreting the new images. This AI tool, along with training and certification, helps radiologists transition from ultrasound alone to OA/US imaging.
The system is indicated for use by trained and qualified healthcare providers to evaluate palpable and non-palpable breast abnormalities in adult patients who are referred for diagnostic imaging breast work-up following clinical presentation or other imaging examinations such as screening mammography.
For more information: www.SenoMedical.com
Reference: [i] Vlahiotis A, Griffin B, Stavros AT, Margolis J. Analysis of utilization patterns and associated costs of the breast imaging and diagnostic procedures after screening mammography. Clinicoeconomics Outcomes Res 2018;10:157-167.


 July 29, 2024
July 29, 2024 








