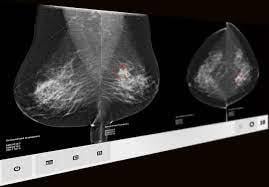
May 3, 2023 — ScreenPoint Medical announced today that its Transpara breast AI has surpassed 4 million mammograms (2D and 3D) analyzed in support of radiologists. The company will highlight Transpara - FDA cleared and with European regulatory approval (CE Mark) for use with 2D mammography (FFDM) and digital breast tomosynthesis (DBT) - at the SBI Breast Imaging Symposium, May 4-6 in Maryland (Booth #407).
Widely used in Europe and the US, the milestone of 4 million mammograms analyzed was reached at Denmark's Capital region screening program. Averaging almost 75,000 mammograms a year analyzed, the screening program is the heaviest global user of Transpara. In 2022, a team led by Ilse Vejborg, MD, Head of the Copenhagen Screening program, demonstrated Transpara's value as an autonomous first reader for 70% of studies (low risk), improving double-reading capacity for cases with higher risk.
President of University Radiology Group (URG, heaviest US-based user of Transpara), Roger Yang, MD, FACR, has specifically talked about Transpara's value in supporting reading workflow by improving focus during the complex mammography reading process and providing confidence.
"Our goal all along has been to improve detection and support improved care for women. As we pass 4 million mammograms analyzed in more than 30 countries, we are delivering on this promise," said founder Professor Nico Karssemeijer.
Designed to assist radiologists with the reading of 2D and 3D mammography exams, Transpara provides radiologists with a 'second pair' of eyes helping detect cancers earlier and reduce recall rates.
CEO Mark Koeniguer sees customers value: "Utilization by customers is the best evidence we have of a real clinical difference. We improve the algorithm and add new features on an annual basis. We are looking forward to continuing to add to our Breast AI Suite."
For more information: https://www.screenpoint-medical.com


 December 10, 2025
December 10, 2025 









