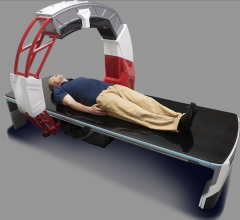
Siemens Medical Solutions’ new dual source computed tomography system has received clearance by the FDA, the company announced during this week's Radiological Society of North America annual meeting in Chicago.
Somatom Definition, which features two X-ray sources at two different energy levels in simultaneous spiral scanning, allows clinicians to explore various scenarios for tissue characterization.
Earlier attempts at dual-energy CT created two subsequent but separate scans at different tube voltages, but failed to seamlessly align the imaged anatomy. Somatom Definition overcomes this limitation by permitting the use of two sources at two different kilovolt levels simultaneously. The result is two spiral data sets acquired in a single scan, which enables clinicians to distinguish the imaged tissue and material.
The device is being also marketed as a solution for direct subtraction of bone in complicated anatomical regions, virtual un-enhanced liver images without multiple scans, evaluation of lung perfusion defects, visualization of cartilage and differentiation between hard plaques and contrast agents.


 August 09, 2024
August 09, 2024